2013 Volume 53 Issue 9 Pages 1594-1598
2013 Volume 53 Issue 9 Pages 1594-1598
In order to improve the coke combustion efficiency during iron ore sintering, the effect of coke granule structure was investigated with a TG-DTA and a new combination heating equipment capable of establishing similar heating pattern to the actual sintering bed. The results were analyzed as a function of granule type and neighboring specie as follows:
The granule type was crucial to the combustion behavior of granules. On the other hand, the effect of neighbors was distinct over core coke particles; meanwhile, the effect remained uncertain in pellet type granules. The authors recommend composite type granules (fine coke particles adhering on a core particle) for the best choice. The type should bring 1.2% save of coke consumption and 2.3% increase in the heat front speed in comparison to coke particles of single type.
Sintering is the major iron-ore agglomeration process working in Japan, which consumes 50 kg/t-sinter cokes and emits 1700 Nm3/t-sinter waste gas. The waste gas contains 1% CO with 10–15% oxygen. The fact implies a chance of improving coke consumption if the perfect combustion comes true.
Coke gasification/combustion phenomena are well known in case of single sphere.1) Embodying the knowledge, so-called Muchi model2) has developed heat wave calculation, which successfully explained the effects of coke content, gas velocity, etc. on heat waves. Actually, the real coke particles react under the influence of granule structure; namely, coke particles exist very close to other kinds of particulates (neighbors) in granules that must cause some complicated effect on the coke combustion.
Little has been studied regarding the effect of granule structure. Hida et al.3) classified granule type and found, using modelled granules made of coke and alumina, that nuclei coke particles increased the combustion rate as the adhesion layers thereon became thinner, and that fines cokes increased it as their concentration in the adhesion layer became denser. Kasai et al.4) followed them to examine the effect of various materials as adhering fines on nuclei coke particles and found that the coke combustion rate decreased as the materials fused at the higher temperature. Since any of the two and others5,6,7) focused on the behaviour of NOx generation, none disclosed enough information on combustion efficiency that was one of objectives of this research.
From energy point of view, it may be practical to identify the phenomena as the bipolar reactions occurring concurrently: one is the exothermic oxidation where coke reacts with the oxygen coming from ambient airflow; the other is the endothermic reduction of iron oxide where coke reacts with adjacent iron ore particles and can be a major producer for CO emission.
We aim at clarifying the criteria that divides the above-mentioned two routes of coke reaction and reducing CO emission by controlling granule structure and preventing the latter reaction. To achieve this, we addressed here two experiments: TG-DTA analysis for measuring ignition temperature and combustion experiment in single layer packed bed for determining combustion rate and efficiency with respect to granule structure and neighbor specie. The latter equipment was capable of establishing similar heating pattern to the actual sintering bed to allow sample granules to ignite freely and naturally. This condition, discriminating ours from the former researches that forced coke granules to ignite at a fixed temperature, expectedly gives us an answer to ‘what granule type is best?’ in a direct and convenient way.
Hida et al.3) examined granule structure of sintering feed and classified into three types: ‘C’, ‘P’ and ‘S’. ‘C’ type was for granules consisted of nuclei particles (>1 mm) and fine particles (<0.5 mm) adhering on the nuclei; ‘P’ type was for agglomerates of fines only; ‘S’ type was for lone particles (0.5–1 mm).
Figure 1 illustrates each type of granule with the corresponding sample cord. The cords of P, C and S have the same meaning as the Hida’s classification and accompany original suffixes: the suffix C and X designate coke and neighbor specie, respectively; A, H, O and L represent alumina, hematite, iron ore (Carajas: A Brazilian hematite iron ore containing 65.5, 0.9, 0.8, 1.2 mass% in T.Fe, SiO2, Al2O3, CW, respectively) and limestone in place of X, respectively; in case of C type, the suffix on the left of hyphen stands for the nucleus particle; the right, for adhering fines.

Granule classification and naming according to Hida et al.3)
According to the Hida classification, we designed three types of granules in the interest of coke as listed in Table 1. Source cokes were sieved out of plant coke breeze into two size splits: –0.25 for adhering fines and 2.0–2.7 mm for cores. Coke-centered granules (Cc-x) were made of 2.0–2.7 mm coke particles covered with hematite reagent or –0.25 mm limestone with the weight ratio of coke to hematite/limestone being 9 to 1. Coke-adhered granules (Cx-c) had –0.25 mm coke fines on nuclei particles of 2.0–2.7 mm iron ore or 2 mm alumina ball with the weight ratio of core to coke fines being 9 to 1. Pellet-type granules (Pcx) were the 1:1 mixture of –0.25 mm coke fines and neighbors of hematite reagent, α-alumina reagent or –0.25 mm limestone.
| Type | Coke (C) | Neighbors (X) | Weight Ratio (C : X) | |
|---|---|---|---|---|
| Size (mm) | Specie | Size (mm) | ||
| SC | 2.0–2.7 | – | – | 1:0 |
| CC-X | 2.0–2.7 | Hematite Limestone | Reagent –0.25 | 9:1 |
| CX-C | –0.25 | Iron ore Alumina ball | 2.0–2.7 2 | 1:9 |
| PCX | –0.25 | Hematite Limestone α-alumina | Reagent –0.25 Reagent | 1:1 |
The ingredients for C- or P-type granules were granulated in a petri dish with spraying water without binder except for the case of coke-alumina combination with an organic binder solution. In case of P-type, the granules that had passed between 2.0 and 2.7 mm proceeded to the combustion test. Every sample stayed in a 383 K oven for a night to dehydrate in advance.
2.2. TG-DTA for Ignition TemperatureTo determine ignition temperatures, Tig, a TG-DTA (TG-DTA2000SA, Burker AXS) operated at the heating speed of 20 K/min under air flow. The equipment had a platinum cell (5 mm in diameter and 5 mm in depth) accommodating four granules of sample.
Figure 2 shows an example of TG-DTA curves and how to determine ignition temperature from them. We read an ignition temperature at the intersection (T3) of the preceding gradually increasing line and the proceeding rapidly increasing one of a TG-DTA curve. Another candidate that a TG curve could offer (T1) was discarded because DTA showed more distinct knick than TG.

An example of TG-DTA curves which explains how to determine the ignition temperature as T3.
Runs at a condition repeated five times; the middle three of five averaged to be the representative value.
2.3. Gas-Image Combination Heating Equipment for Combustion Rate and EfficiencyFigure 3 shows a new assembly to examine the single-layer combustion behavior of coke granules. A 10 mm-dia. sample vessel, accommodating six sample granules on ceramic wool, located in a T-shaped 40 mm-dia. quartz tube. Over the vessel an infrared lamp emitted hot beam and gas heater simultaneously supplied hot air of 1273 K at 5 NL/min from an end of the tube to establish a quick heating condition similar to the heat front of sintering bed. From the bottom of vessel a high speed gas analyzer sucked 1.5 NL/min combustion gas to determine CO, CO2 and O2 concentration. The excess hot air overflowed from an outlet at another end of the tube.
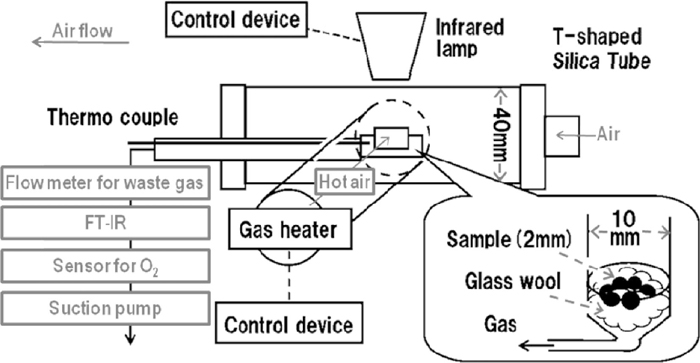
The new assembly for combustion experiment to determine the combustion rate and efficiency.
Figure 4 is an example of gas analysis result, which confirms that the ambient temperature around the vessel quickly elevated over 1273 K. The coke combustion rate, Rc, was defined as the quotient of carbon weight charged in sample divided by combustion time that corresponds to the width at half height of CO peak. The combustion efficiency, Ec, was also defined as the ratio of CO2/(CO + CO2) where each gas volume generated during experiment was digitally summed from each logging datum.

An example of combustion experiment which explains how to determine the combustion rate. The origin of ‘Time’ axis is the point putting on the suction pump.
Combustion of coke particles in granules relates to individual size, position in granule and character of neighbors. Since the position is dependent on the size in sintering granules, they are usually treated as a single factor of granule structure (type).
Figure 5 shows the result on Tig, Rc and Ec with respect to granule type and neighbors, where CC-X is also regarded as a Sc type with neighbors. The granule type affected distinctly combustion behavior as Tig, Rc and Ec ranked SC > CX-C > PCX > CC-X, PCX > CX-C > SC ~ CC-X and CX-C > PCX > SC > CC-X, respectively in order of decreasing. The effect of neighbors was distinct for Sc (in CC-X) in a way that both of limestone and hematite decreased Tig and Ec; meanwhile, the effect was uncertain in PCX.

Results. The effect of granule type on (a) ignition temperature, (b) combustion rate and (c) combustion efficiency+) in comparison with Hida et al.3) *) CO-C indicated an abnormally low value possibly due to ore bursting.
The structural effect was superior to the neighbor; therefore, we will discuss the former effect first. The discussion on neighbor effects will follow within the restricted type of Sc (CC-X).
3.1. Effect of Granule StructureCC-X type decreased in Tig and Ec using SC type as a reference. The effect must come from hematite and limestone covering over coke core particles, which will be revisited latter in §3.2 as a neighbor effect.
CX-C type coke decreased in Tig and increased Rc and Ec. This effect must be contribution of particle size only. The variations in Tig and Rc due to particle size are well known as combustion behaviors of a single sphere: Semenov approach to thermal ignition8) explains the Tig lowering; simulation with a shrinking core model predicts the Rc acceleration for smaller particles. Igniting at lower temperature and burning for shorter time should lower the burning temperature of coke particles on average, which can improve Ec by the thermodynamic mechanism that the reaction of C + CO2 = CO is slower than that of C + O2 = CO2 at the lower temperature.
PCX type unchanged in Tig, increased in Rc, decreased in Ec, comparing with CX-C, Supposing that Tig is subject to the reaction on surface of granule, the coincidence in Tig is reasonable, since coke particles on surface were of the same size and in the similar situation to interact with the air outside. The change in Rc and Ec can reflect the experimental condition that a granule of PCX had five times coke in weight than for CX-C, which caused the increase in combustion temperature, resulting in the change as the same thermodynamic mechanism mentioned above.
Figure 5 includes Hida’s observations on Rc and Ec, which are consistent with ours on the superiority of CX-C and PCX to SC and CC-X in Rc and Ec. According to the Hida’s observations Rc and Ec in PCA coincide with respective values in CA-C, which is probably owing to their experimental condition that they used the same carbon contents for CA-C and PCA and the condition brought the same combustion temperatures to CA-C and PCA despite of this work. Additionally, we guess the bias in the data between this work and Hida came from such difference in experimental conditions that Hida et al. burned coke granules as a packed bed (22 mm in diameter and 50 mm in height) that had been preheated to 1073–1473 K in an electrical furnace in under nitrogen atmosphere.
3.2. Effect of Adhering Neighbors on Combustion of Coke CoreThere were significant effects of adhering neighbors on the combustion characters of coke core, which were comparative with respect to species: limestone and hematite.
Tig distinctly decreased both in CC-H and CC-L. Hematite and limestone can behave as oxidizer, i.e. C + Fe2O3 → CO + 2FeO and C + CaCO3 → 2CO + CaO, respectively, and their close contacts to carbon will facilitate such reactions to lowered the Tig. In case of hematite many9,10,11) have reported that close contact of coke particles initiate reducing reaction of hematite at lower temperature in the field of carbon-iron composite pellets; such acceleration expectedly occurs also in case of limestone.
The decreases of Rc were insignificant in both cases comparing to SC. Kasai et al.4) had examined various kinds of coverage over coke and found all decreased Rc but CF case, discussing that the exception came from a slag-off effect of CF. We suppose that the similar slag-off phenomena occurred and maintained the RC in our cases with support from the following observation:
There appeared a residue in round-shaped grains after combustion, implying the occurrence of some melt formation. Intensive point analysis of the grains with SEM/EDS revealed their compositions, which Figs. 6(a) and 6(b) show in FeO–SiO2–Al2O3 system for hematite and in CaO–SiO2–Al2O3 system for limestone, respectively. Most points located on the line linking between the original compositions of hematite or lime and coke ash. The fact substantiated that the hematite or lime had reacted with coke ash to form slag of low melting point.

Plots of residue compositions on (a) CaO–SiO2–Al2O3 system for CC-L and (b) FeO–SiO2–Al2O3 system for CC-H.
EC were slightly decreased in both cases. The above-mentioned reactions that lowered Tig can be an explanation for the fact. Since the reactions produce CO, the CO gas enriches CO concentration of combustion gas.
The difference from PCA to PCH or PCL remained uncertain, which requests further experiments before discussion on the effect of hematite or limestone in granules of PCX type.
3.3. Estimation of Effects on Sintering Heat WaveWe recommend CX-C type for the sustainable choice in iron ore sintering process based on the results on Ec, where Ec gains 0.12 from 0.50 for SC to 0.62 for CO-C. This effect accounts for 1.2% save of total coke consumption as the imperfect combustion loses approximately 10% of calorific value of coke (Δ1.2% = Δ0.12 × 10%).
The CO-C can also enhance the productivity of sintering machine. Nakano et al.12) has offered an equation to estimate heat front speed as v(1 + 576/Tig). Based on the Tig change from 979 K for SC to 947 K for CO-C, the effect accounts for 2.3% increase in the heat front speed.
Aiming to improve the coke combustion behavior during sintering, we measured Tig by TG-DTA and determined Rc and Ec with a combination-heating equipment capable of establishing a heating pattern similar to that of actual sintering bed. The results were analyzed as a function of granule type and neighboring specie as follows:
(1) The granule type was crucial to the combustion behavior of granules. Tig, Rc and Ec ranked SC > CX-C > PCX > CC-X, PCX > CX-C > SC ~ CC-X and CX-C > PCX > SC > CC-X, respectively in order of decreasing.
(2) The effect of neighbors was distinct for Sc (in CC-X) in a way that both of limestone and hematite decreased Tig and Ec; meanwhile, the effect was uncertain in PCX.
(3) The results recommend CX-C type for the best choice. The type will bring 1.2% save of coke consumption and 2.3% increase in the heat front speed in comparison to SC type.