2013 Volume 53 Issue 9 Pages 1607-1616
2013 Volume 53 Issue 9 Pages 1607-1616
The substitution of charcoal as an alternative fuel to coke breeze in a simulated Japanese Steel Mills (JSM) sinter blend was investigated. Compared with coke breeze, higher mix moisture contents were required for the sinter mixture containing charcoal to achieve optimum granulation. The green granules formed from the sinter mixture containing charcoal were clearly less dense and formed a less compacted green bed as evidenced by the packing density. To achieve return fines balance, fuel addition had to be increased from 3.62 to 4.17% (on a dry mixture basis) as the substitution of charcoal increased from 0 to 50%. However, at 100% subsitution, the sinter mixture failed to achieve balance even at a very high fuel addition level of 4.7%. Compared with the sinter fired with coke breeze, the sinter from the mixtures containing up to 50% charcoal was marginally weaker in terms of sinter yield, tumble strength (TI) and reduction disintegration (RDI). The reasons for weaker sinter are discussed. Fuel rate increased considerably with charcoal substitution due to increased fuel addition and decreased sinter yield. However, increasing fuel rate did not lead to a reduction of sintering productivity. In contrast, the sintering speed and productivity were maintained as the charcoal substitution rate increased from 0 to 25% and then increased considerably with further increase in charcoal substitution rate. The emission mechanisms of the CO, CO2, SO2 and NOX and H2O gases during sintering are clearly quite different. CO, CO2 and NOx emission was observed over the entire sintering process and varied slightly as the sintering process progressed. However, the SO2 and H2O emissions were observed only towards the completion of the sintering process. Both the CO and CO2 concentrations in the waste gas increased with the increasing substitution of charcoal for coke breeze; however the concentrations of SO2 and NOX in the waste gas decreased.
Iron and steel production accounts for about 70% of world greenhouse gas emissions generated from primary metal production.1) While there are a number of short term approaches to reducing CO2 emissions from iron and steel production, the longer term approach will require gradual substitution of fossil fuel-based energy and reductants such as coke and coal with renewable energy sources such as biomass materials.1) In this way carbon is recycled through the atmosphere as CO2 in a 5–30 year cycle compared with fossil carbon which was deposited in geological time. Charcoal is also much lower in sulphur and nitrogen, hence lowering SOX and NOX emissions. However, the challenges are:
• to develop and manage the renewable sources on a sustainable basis;
• to develop a high capacity production technology that produces charcoal from the renewable sources at a low cost and low environmental impact; and
• to achieve the qualities ideally suited to product applications, such as iron ore sintering and blast furnace ironmaking.
There is an ongoing collaboration between BlueScope Steel, OneSteel and CSIRO to investigate ways to substitute charcoal, derived from biomass, for pulverised coal and coke to reduce net CO2 emissions from existing operations.2) Previous experimental and plant-based work has shown that charcoal is a feasible alternative to coal in many steelmaking operations. Somerville et al.3,4) have demonstrated that specially prepared charcoal is a viable alternative to calcined anthracite for recarburising liquid steel, although issues of moisture absorption and low density remain. In mathematical modelling and experimental studies, Mathieson et al.5,6) showed that charcoal can be a viable alternative to coal as a blast furnace tuyere injectant.
However, limited work7,8,9,10) has been conducted to investigate the application of biomass in the sintering process to replace coke breeze, with this work mainly focused on its enviromental impacts and at low substitution rates. In the previous studies,7,9) substitution of charcoal for coke breeze was reported to result in higher sintering productivity, but with much higher fuel rates and weaker sinter. The work by Lovel et al.7) also showed considerable reductions in SOx and NOx when charcoal was used as fuel. The recent CSIRO results11) have demonstrated that it is possible to achieve return fines balance and sinter quality, while maintaining high sintering productivity, by fine tuning the propoerties of charcoal. This paper aims to give an in-depth investigation of the effect of increasing substitution of charcoal on the granulating, sintering and emission characteristics of a simulated JSM sinter blend.
Table 1 summarises the chemical analyses of the component ores used in the base ore blend, which was based on typical JSM sinter blends. As shown in Table 1, the base ore blend is quite low in SiO2 (3.71%) and Al2O3 (1.48%), and moderately high in Loss on Ingition (LOI) (5.13%). The base ore blend consisted of a balanced mixture of ore types from very dense to moderately microporous Brazilian hematitic ores, to moderately and highly microporous, reactive Australian Marra Mamba and pisolitic ores. The size distribution of the base ore blend contained 42% +2 mm material as potential nucleus particles and 26% –0.25 mm material as potential adhering fines. As a result, it had a quite coarse mean size of about 2.5 mm. Overall, the base ore blend was designed to perform well in granulation and sintering due to its favourable chemical, physical and mineralogical characteristics.
| Ore | FeTotal | SiO2 | Al2O3 | P | LOI total | CaO | MgO | H2O |
|---|---|---|---|---|---|---|---|---|
| Ore 1 | 67.07 | 0.94 | 0.93 | 0.019 | 1.40 | 0.02 | 0.02 | 4.58 |
| Ore 2 | 65.78 | 3.47 | 0.73 | 0.027 | 0.86 | 0.14 | 0.03 | 1.83 |
| Ore 3 | 61.73 | 3.14 | 2.05 | 0.062 | 5.88 | 0.05 | 0.02 | 3.62 |
| Ore 4 | 61.48 | 3.43 | 2.08 | 0.062 | 6.18 | 0.02 | 0.08 | 7.39 |
| Ore 5 | 63.43 | 4.09 | 2.04 | 0.062 | 2.46 | 0.1 | 0.1 | 4.81 |
| Ore 6 | 62.44 | 3.57 | 2.03 | 0.070 | 4.36 | 0.07 | 0.06 | 2.87 |
| Ore 7 | 57.96 | 5.41 | 1.38 | 0.037 | 9.66 | 0.07 | 0.06 | 5.60 |
| Base Ore Blend | 62.22 | 3.71 | 1.48 | 0.04 | 5.13 | 0.07 | 0.05 | 4.51 |
A commercial red gum charcoal was obtained as a biomass source for the preparation of potential renewable fuels for the subsequent sintering tests. The proximate and ultmate analyses of the red gum charcoal are compared in Table 2 with the properties for the coke breeze used. Preliminary tests found that the red gum charcoal contained a quite high content of volatile matter and was not able to achieve sinter quality at high substitution rates for coke breeze.11) Therefore the red gum charcoal was further devolatised at 650°C in a N2 atmosphere using a modified rotary furnace. The treated charcoal sample was then crushed using a roller crusher to simulate the size distribution of coke breeze available. However, it is impossible to effect a size reduction of charcoal in the same way as for coke breeze, as a consequence of the soft, fibrous nature of the charcoal. As shown in Table 2, the treated charcoal sample had a narrower size distribution, containing more –4+0.25 mm material and less +4 mm coarse and –0.25 mm fine materials compared with the coke breeze. The proximate and ultmate analyses of the treated charcoal sample are also listed in Table 2. The volatile matter of the treated charcoal sample was reduced to 8.2% from 19.9%. Compared with coke breeze, the treated charcoal sample was lower in ash, N and S contents, but with a higher volatile matter content and calorific value. The treated charcoal sample also showed a very high moisture saturation value of 48.3%, roughly twice that of the coke breeze (25.4%), which is indicative of the extremely porous nature of the treated charcoal sample. Therefore, substitution of the treated charcoal sample for coke breeze is expected to increase the mix moisture content required for achieving efficient granulation as this depends on the amount of surface moisture added beyond the saturation of the internal pores.
| Red Gum Charcoal | Treated Charcoal | Coke | ||
|---|---|---|---|---|
| Pyrolysis temperature | °C | 650 | ||
| Proximate analysis | ||||
| Moisture | %ad | 6.6 | 5.4 | 0.8 |
| Ash | %ad | 2.6 | 4.0 | 13.2 |
| Volatile matter | %ad | 19.9 | 8.2 | 1.4 |
| Fixed carbon | %ad | 70.9 | 82.4 | 84.6 |
| Ultimate analysis | ||||
| C | %daf | 83.4 | 91.6 | 95.7 |
| H | %daf | 2.38 | 1.49 | 0.36 |
| N | %daf | 0.29 | 0.41 | 1.42 |
| S | %daf | 0.06 | 0.06 | 0.48 |
| O | %daf | 13.8 | 6.4 | 2.1 |
| Size distribution | ||||
| 4 | mm | 1.3 | 13.4 | |
| –4+0.25 | mm | 78.7 | 60.1 | |
| –0.25 | mm | 20.0 | 26.6 | |
| Moisture saturation | % | 48.3 | 25.4 | |
| Calorific value | MJ/kg (ad) | 28.34 | 30.58 | 28.48 |
The granulating characteristics of the JSM sinter blend containing the coke breeze and treated charcoal were studied by making up a number of sinter mixtures with different water additions and fuel types from the component raw materials and measuring the permeability of a packed, green granule bed. The effect of mix moisture and fuel type on the granulating characteristics of the JSM sinter blend was investigated.
The sintering performance of the JSM sinter blend containing different fuel types was established at a bed diameter of 300 mm and a bed height of 600 mm using a pilot-scale, pot-grate sintering facility, as shown in Fig. 1. The granulated sinter mix was charged onto a layer of hearth material, approximately 30 mm thick, consisting of sinter particles of –16+10 mm supported on a thick stainless steel grate bar. The coke breeze at the top surface of the sinter mixture was ignited for 90 s at a constant suction of 8 kPa by multiple propane burners. At the end of the 90 s ignition cycle, the propane burners were switched off and the suction across the sinter bed was ramped up and maintained at 16 kPa to move the flame front (the layer of burning coke breeze) downwards. The sintering process finishes when the flame front reaches the layer of hearth material. After being cooled, the fired sinter plugs were processed using the CSIRO combined drop tower/jaw crusher procedure and the size distribution of the sinter particles was determined using a set of trommel screens. Based on the data recorded, the sintering productivity and fuel rate, sinter yield, and return sinter fines balance for each firing test were then calculated. The sinter particles were further sampled for determination of sinter chemistry and quality indices, including TI (tumble index measured in accordance with ISO 3271:2007), RI (reduction index measured in accordance with ISO7215:2007) and RDI (low-temperature reduction-disintegration index measured in accordance with ISO4696-2:2007). Table 3 summarises the sintering test conditions applied and sinter chemistry targets.
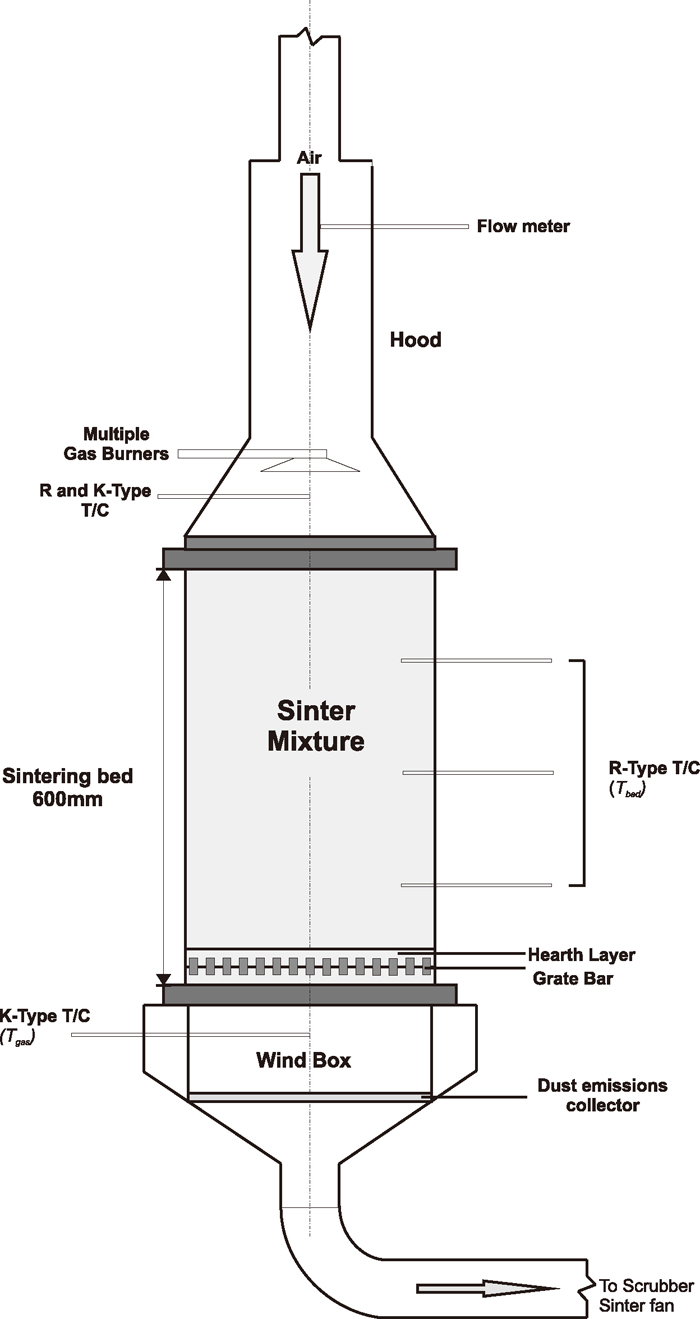
Schematic diagram of the pilot-scale pot-grate sintering facility.
| Parameters | Sintering Conditions | |
|---|---|---|
| Bed height (total) | mm | 600 |
| Return fines (dry mix basis) | % | 23 |
| Return fines sizing | mm | –5 |
| Hearth layer depth | mm | 30 |
| Hearth layer size | mm | –16 + 10 |
| Ignition flame temp | °C | 1300 |
| Ignition time | s | 90 |
| Ignition suction | kPa | 8 |
| Sintering/cooling suction | kPa | 16 |
| Basicity (CaO/SiO2) | kg/kg | 1.9 |
| Sinter SiO2 level | % | 4.8 |
| Sinter MgO level | % | 1.6 |
For selected sintering tests, a set of thermocouples was pre-positioned in the sinter mixture at various depths of the sinter bed and in the wind box to monitor the progression of the heat front through the sintering bed and off-gas temperature. The location of these thermocouples is shown schematically on the test facility in Fig. 1. Air flow measurements were made using an annubar flowmeter in the inlet duct and a pitot tube in the outlet gas passage.
2.4. Waste Gas Composition MeasurementThe concentrations of CO2, CO, SO2, NOX, and moisture in the waste gas were measured using Fourier-transform infrared spectrometry (FTIR). Gas samples were extracted continuously from the waste gas duct via a stainless steel probe and passed through a heated sample line maintained at 180°C and an ADI heated head diaphragm sampling pump into a temperature controlled sample cell of the Gasmet DX4000 FTIR spectrometer. The O2 concentration of the waste gas was measured using an Oxigraf oxygen sensor which uses laser absorption spectroscopy to measure the concentration of oxygen molecules in a gas sample.
The granulating characteristics of the JSM sinter blend containing different types of solid fuel, plotted as JPU permeability curves, are shown in Fig. 2(a). The blends had similar permeability versus mix moisture profiles and achieved maximum permeability at specific moisture contents. The optimum moisture content required for efficient granulation was approximately 7.05% for Blend 1 (with coke breeze), much lower than the mixture containing the treated charcoal sample (8.45%). Substitution of the treated charcoal for coke breeze in the base blend shifted the permeability curve towards the right and increased the moisture requirement for optimum granulation. This agreed with the moisture saturation measurement, showing that the treated charcoal sample had a much higher moisture saturation value. Therefore, the blend containing the treated charcoal sample required a higher mix moisture content in subsequent pot-grate sintering tests.
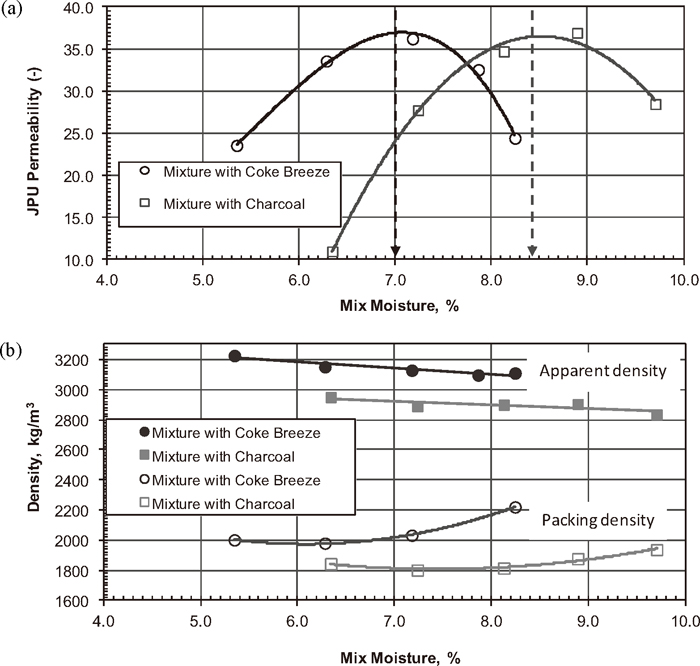
(a) Permeability calculated in JPU and measured at a pressure drop of 6 kPa across the green granules and (b) apparent and packing densities of green granules prepared from the JSM blend containing different types of solid fuel.
The apparent and packing densities of green granules made from the JSM blend containing different types of solid fuel were also measured and are shown in Fig. 2(b). The green granules formed from the sinter mixture containing the treated charcoal were clearly less dense and formed a less compacted green bed as evidenced by the packing density.
3.2. Effect of Charcoal Substitution on Sintering CharacteristicsTable 4 summarises the granule characteristics and the pre-ignition airflow across the green granules of the sinter mixtures with increasing substitution rate of charcoal for coke breeze. To maintain granululation efficiency and therefore pre-ignition airflow across the sinter mixture, the mix moisture content was increased with the substitution rate of charcoal for coke breeze. The pre-ignition airflows are comparable between the sinter mixtures, although the mean diameter of green granules decreased slightly. However, the apparent and packing densities of green granules decreased considerably as the substitution rate of charcoal increased.
| Charcoal substitution rate | Mix moisture content | Sauter mean diameter of green granules | Apparent density of green granules | Packing density of green granules | Pre-ignition air flow at 6 kPa | Pre-ignition air flow at 16 kPa |
|---|---|---|---|---|---|---|
| % | % | mm | kg/m3 | kg/m3 | m3/min | m3/min |
| 0 | 6.76 | 1.90 | 3189 | 1904 | 2.50 | 3.83 |
| 25 | 6.76 | 1.89 | 3092 | 1885 | 2.24 | 3.80 |
| 50 | 7.15 | 1.85 | 3051 | 1817 | 2.19 | 3.78 |
| 100 | 7.86 | 1.77 | 2898 | 1696 | 2.31 | 3.87 |
One of the key objectives of pot-grate sintering tests is to achieve a balanced return fines outcome, where the amount of returns generated is equal to the amount of returns added in the sinter mixture. Figure 3 shows the fuel addition required to achieve the balance and sinter quality for the sinter mixtures with increasing substitution of charcoal for coke breeze. As the substitution of charcoal increased, the ratio of returns generated to the returns added (RFB) increased slightly, suggesting slightly deteriorating sinter yields due to the weakening of sinter plugs for the sinter mixtures with high substitution rates of charcoal. To achieve balance, the fuel addition had to increase from 3.62 to 4.17% as the substitution of charcoal increased from 0 to 50%. However, at 100% subsitution, the sinter mixture failed to achieve balance even at a very high fuel addition level of 4.7%.
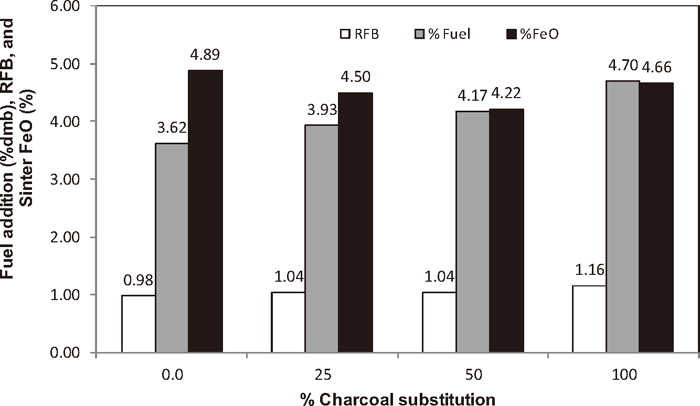
Fuel addition required and RFB and sinter FeO achieved for the sinter mixtures with increasing substitution of charcoal for coke breeze.
Figure 4 shows the sintering performance and sinter quality of the mixtures with increasing substitution of charcoal from 0 to 50%. The sintering performance of the mixture containing 100% charcoal was not included in the discussion as it failed to achieve balance. As charcoal substitution led to increased fuel addition and decreased sinter yield, the fuel rate increased considerably with charcoal substitution rate, as evidenced in Fig. 4(a). However, increasing fuel rate did not lead to a reduction of sintering productivity, which is often in an inverse proportion to the fuel rate. In contrast, the sintering speed and productivity were maintained as the charcoal substitution rate increased from 0 to 25% and then incresed considerably with further increase in charcoal substitution rate. As shown in Fig. 4, compared with the sinter fired with coke breeze, the sinter from the mixtures containing up to 50% charcoal in substitution for coke breeze was only marginally weaker in terms of sinter tumble strength (TI) and reduction disintegration (RDI). The sinter from sinter mixtures containing up to 50% charcoal achieved the minimum quality required for pot-grate sinter.

Sintering performance and sinter quality of sinter mixtures with increasing charcoal substitution from 0 to 50%.
Figure 5 shows the temperature profiles measured at the top, mid-height and bottom of the sintering bed during a typical sintering test using coke as fuel. The maximum bed temperature attained at the top of the sintering bed was found to be above 1280°C, which is much higher than the formation temperature of an initial sinter melt. It is generally believed that the initial sinter melt starts to form approximately at 1100°C, and then reacts progressively with nucleus particles nearby until the reaction zone temperature reaches ~1100°C, below which the sinter melt solidifies. The time over which the reaction zone temperature is maintained above 1100°C is important. A longer retention time means more time for nucleus assimilation and consolidation of pores and sinter liquid, leading to a better sinter structure. As shown in Fig. 5, both the maximum bed temperature and retention time increased as the sintering process progressed. As no fuel segregation was intended, coke breeze was believed to have been distributed evenly across the height of the sinter bed. Therefore, less heat loss occurred as the sintering process progressed. As a consequence, the sinter fired at the bottom was stronger than the sinter fired at the top of the sinter pot. While the temperature profiles measured at different depths within the sinter bed in Fig. 5 are similar, the rise in bed temperature delayed slightly from the top to bottom sections of the sinter bed, indicating that the heat transfer rate slowed down slightly as the sintering process progressed.

Temperature profiles measured at the top, mid-height and bottom of a sintering bed and in the wind box during a typical pot sintering test on a sinter mixture containing 100% coke breeze.
Figure 6 shows the temperature profiles measured at the mid-height of the sinter bed and wind box for sinter mixtures containing increasing substitution of charcoal for coke breeze. As shown in Fig. 6, by increasing the fuel addition, the sinter mixtures containing various substitution rates of charcoal achieved the maximum bed temperature and retention time at either comparable or better temperatures and times compared with the sinter mixture containing 100% coke breeze. However, as the charcoal substitution rate increased, the sintering time decreased considerably.

In-bed temperature profiles measured at the mid-height of sinter bed and wind box for the sinter mixtures with increasing charcoal substitution.
Typical composition ranges of waste gases from underneath an industrial sinter strand are reported to be in the range of 2–16% CO2, 1–3% CO and 8–15% O2.12) The CO, CO2 and O2 concentrations evolved during the sintering of sinter mixtures with different charcoal substitution rates are shown in Fig. 7. During the steady progress of the sintering process, the O2 concentration was more or less constant and the CO concentration increased gradually, but the CO2 concentration decreased. As mentioned earlier, less heat loss occurred as the sintering process progressed. Consequently, the flame temperature increased, which shifted the CO/CO2 equilibrium towards CO formation. Therefore, the CO concentration in the waste gas increased and the CO2 concentration in the waste gas decreased as the flame front moved, indicating less efficient combustion at the bottom of the sinter pot.

CO and CO2 and O2 concentrations in the waste gas measured for sinter mixtures with increasing charcoal substitution.
While the CO and CO2 evolution traces are similar for the sinter mixtures with different charcoal substitutions, the waste gas compositions are very different, as evidenced in Fig. 7. Both the CO and CO2 concentrations in the waste gas increased with the increasing substitution of charcoal for coke breeze, however the concentration of O2 in the waste gas decreased. As shown in Fig. 3, the fuel addition was increased with substitution of charcoal for coke breeze to achieve balance and sinter quality. Therefore, more oxygen was consumed during combustion at higher charcoal substitutions, resulting in a lower O2 concentration in the waste gas.
Figure 8 shows the concentration of NOX, SO2 and water vapour emitted during the sintering of mixtures with increasing charcoal substitutions. The emission mechanisms of these gases during sintering are clearly quite different. NOx emission was observed over the entire sintering process and decreased slightly as the sintering process progressed. However, the SO2 and H2O emissions were observed only towards the completion of the sintering process. During the sintering process, water moved down the bed through a sequence of water evaporation and condensation cycles as the flame front moved downwards. As the result of these evaporation and condensation cycles, layers of calcined granules and wet granules were formed ahead of the combustion zone and moved downwards with the flame front. The water trapped in the wet layer was eventually emitted as a peak at the end of sintering process. Since SO2 is quite soluble in water, it also moved through the sinter bed with the water, exhibiting a peak concentration as the burn-through point is approached. Both NOX and SO2 emissions were directly related to the coke combustion, but unlike SO2, NOx has no tendency to be absorbed by the moist colder burden ahead of the flame front and hence its emission is more uniform than SO2 and water vapour throughout the sintering process. As shown in Fig. 8, the NOx concentration rapidly rose after ignition and then reduced slightly over time. Also shown in Fig. 8, both the NOX and SO2 concentrations in the waste gas decreased with increasing substitution of charcoal for coke breeze due to the much lower N and S contents of the treated charcoal sample.

SO2, NOX and H2O concentrations measured in the waste gas for sinter mixtures with increasing charcoal substitution.
While the charcoal sample had a slightly higher calorific value than the coke breeze, the sinter mixtures with higher charcoal substitution rates were found to require more fuel to achieve return fines balance and sinter quality. Compared with coke breeze, charcoal is more porous and is able to absord and hold approximately twice as much water. As a result, additional water is required for granulating the sinter mixture as charcoal is substituted for coke breeze in order to achieve optimum mean granule size and airflow. The amount of energy required during sintering to evaporate the moisture from the sinter mixture into the gas phase increases with increasing substitution of charcoal for coke breeze.
In addition, the CO and CO2 concentrations in the waste gas were found to increase as the substitution of charcoal for coke breeze increased, but the concentration of O2 in the waste gas decreased. The ratio CO2/(CO2+CO) was calculated for sinter mixtures containing 0%, 25%, 50% and 100% coke substitution with charcoal and is shown in Fig. 9. During the sintering process, calcination of fluxes in the sinter mixture, such as limestone and dolomite, generates a considerable amount of CO2. Therefore, the calculated ratio CO2/(CO2+CO) is not a true measure of combustion efficiency. However, since the amount of limestone and dolomite in the sinter mixes remained constant in these experiments, the above ratio still provides a good indicator for comparing the relative combustion efficiency of fuel with increasing charcoal substitution for coke breeze. The CO2/(CO2+CO) ratio during sintering with coke alone as fuel was close to 0.90, and decreased to below 0.8 when sintering with 100% treated charcoal. Hence, the combustion becomes less efficient for sinter mixtures using charcoal substitution for coke. The O2 concentration was also found to decrease considerably with increasing charcoal substitution. This, together with the more permeable sinter bed associated with sinter mixtures containing charcoal, prevents the CO produced at the surface of the burning fuel from being further oxidised to CO2. Hence, increasing fuel addition was required to provide sufficient heat for the sinter mixtures with increasing charcoal substitution for coke breeze.

Combustion efficiency as defined by CO2/(CO+CO2) during sintering of sinter mixtures with increasing substitution of charcoal for coke breeze.
To achieve balance, the fuel addition was increased from 3.62 to 4.17% as the proportion of charcoal in the fuel increased from 0 to 50%. At 100% subsitution of charcoal for coke breeze, the sinter mixture failed to achieve balance even at a fuel addition level of 4.7%. Therefore, there was a clear trend that the ratio of returns generated to the returns added (RFB) increased with the substitution of charcoal for coke breeze even though higher fuel additions were used for the mixtures containing charcoal. A higher return fines ratio often suggests the weakening of bonding of granules in sinter plugs. It is unclear why the same ore blend generates weaker sinter when charcoal is used to replace coke breeze.
In the present study, the apparent and packing densities of green granules were found to decrease considerably as the substitution rate of charcoal increased. Therefore, when the granules from the sinter mixture containing the treated charcoal were packed in the sinter pot, the contact between the granules in the sinter pot and between the particles in the granules were not as close as the more compacted bed of dense granules from the sinter mixture containing coke breeze. Close contact between granules and particles is very important in reducing the pore space and facilitating sinter melt formation via the interactions between mineral particles in the sinter mixture, both leading to a stronger and more coherent sinter which generates less fines.
From the temperature profiles measured at the mid-height of the sinter bed for sinter mixtures containing increasing substitution of charcoal for coke breeze, the sinter mixtures achieved maximum bed temperatures and retention times either comparable or better than those for the sinter mixture containing 100% coke breeze by increasing the fuel addition. However, from the sinter chemistry, the sinter FeO clearly decreased as the substitution of charcoal increased. The sinter FeO content is a good indicator of the maximum temperature the sintering layer is exposed to. Therefore, there is likely to be a decoupling between what was measured by the in-bed thermocouples and what can be derived from the sinter FeO. It is possible that the in-bed thermocouples measured the gas temperature around them, which can be different from the temperature for the sintering layer. The temperature of the sintering layer, as evidenced by the sinter FeO content, is not as high as would be expected.
Figure 10 shows the pressure drops measured across the inlet and outlet flow sensors during sintering for sinter mixtures with increasing substitution of charcoal for coke breeze. While the sinter mixtures showed similar pre-ignition air flow, the airflows measured during sintering are quite different, suggesting different flame front permeability of the sinter mixtures. The flame front of the sinter mixtures is more permeable at higher subsititution rates of charcoal. The highly permeable sintering bed probably resulted in a large proportion of the combustion energy from the flame front being carried away by the waste gas as sensible heat. As a result, the sinter mixtures with higher charcoal substitution rates produced a low temperature flame front, as demonstrated by the low sinter FeO content.
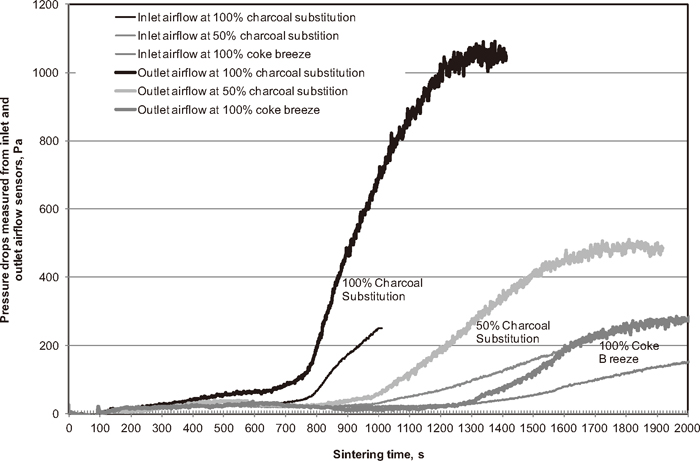
Pressure drops measured across the inlet and outlet airflow sensors.
A low temperature flame front will inevitably reduce the degree of melting and consolidation in the sintering bed, thereby decreasing the degree of bonding between granules and improving the flame front permeability further. As a result, an unbalanced and weaker sinter structure was produced. A narrow flame front is expected to reduce the consolidation time of the sintering bed (leading to a weaker sinter structure) whilst improving the permeability of the flame front during sintering (resulting in a fast sintering process).
The substitution of charcoal as an alternative fuel for coke breeze in a simulated Japanese Steel Mills (JSM) sinter blend was investigated. The effect of increasing substitution of charcoal on the granulating, sintering and emission characteristics of the JSM sinter blend is summarised below:
(1) Compared with coke breeze, higher mix moisture contents were required for the sinter mixtures containing charcoal to achieve optimum granulation. The green granules formed from the sinter mixtures containing charcoal were clearly less dense and formed a less compacted green bed as evidenced by the packing density.
(2) To achieve return fines balance, the fuel addition had to increase from 3.62 to 4.17% as the substitution of charcoal increased from 0 to 50%. However, at 100% subsitution, the sinter mixture failed to achieve balance even at a very high fuel addition level of 4.7%. Compared with the sinter fired with coke breeze, the sinter from the mixtures containing up to 50% was marginally weaker in terms of sinter yield, tumble strength (TI) and reduction disintegration (RDI). The reasons for the weak sinter are discussed above.
(3) Fuel rate increased considerably with charcoal substitution due to the increased fuel addition and decreased sinter yield. However, increasing the fuel rate did not lead to a reduction of sintering productivity. In contrast, the sintering speed and productivity were maintained as the charcoal substitution rate increased from 0 to 25% and then increased considerably with further increases in the charcoal substitution rate.
(4) The emission mechanisms for the CO, CO2, SO2 and NOX gases during sintering are clearly quite different. CO, CO2, NOx emissions were observed over the entire sintering process and varied slightly as the sintering process progressed. However, the SO2 and H2O emissions were observed only towards the completion of the sintering process. Both the CO and CO2 concentrations in the waste gas increased with increasing substitution of charcoal for coke breeze, however the concentrations of SO2 and NOX in the waste gas decreased.
The authors wish to acknowledge the financial and technical support of the project sponsors BlueScope Steel, OneSteel and the CSIRO Minerals Down Under Flagship. The authors also wish to thank the technical teams at CSIRO’s Pullenvale and Clayton laboratories, who helped produce the charcoals and conduct the pilot-scale sinter pot tests.