2013 Volume 53 Issue 9 Pages 1673-1682
2013 Volume 53 Issue 9 Pages 1673-1682
The heat hardening by oxidation is a process commonly used in iron ore pelletization process. The green pellets are fired in induration machine using Corex gas at JSW Steel Limited Pellet Plant. The firing temperature of induration machine strongly influences the physical and metallurgical properties of the pellet. Due to absence of exothermic reaction and poor roasting property of hematite pellet, the energy consumption of hematite pellet production is at higher side and requires higher roasting temperature. In present pelletization process, carbon burdened method is found to be more favorable technique in practice to enhance the induration of hematite pellets. Coke breeze is added in the pellet mix at JSW pellet plants to get the inherent fuel value of a hematite green ball equal to that of a magnetite pelletizing feed. The firing temperature (from corex gas) of the induration machine and carbon addition in the pellet mix is interrelated and decides physical and metallurgical properties of the pellets. At JSW Steel Pellet Plant the carbon addition varies from 0.90 to 1.50% and firing temperature varies from 1230 to 1320°C. Fluctuations in physical and metallurgical properties were observed due to deviation in carbon addition and firing temperature. Optimization of external firing temperature and coke breeze addition in the green pellet mix is necessary to get the desired properties of the pellet for iron making units. Basket trials were carried out at pellet plant induration machine by varying the external firing temperature from 1220 to 1330°C and coke breeze addition from 0.7 to 1.4%. At firing temperature of 1220, 1250, 1280, 1310 and 1330°C the optimum carbon addition 1.30, 1.20, 1.10, 0.90 and 0.70% achieved the optimum physical and metallurgical properties of the pellet for iron making units respectively.
The physical and metallurgical properties like tumbler index (TI), cold crushing strength (CCS), reduction degradation index (RDI) and reducibility of iron ore pellet influence the performance of corex and blast furnace iron making units. The heat hardening by oxidation is a process commonly used in iron ore pelletization process. The pellets are hardened due to re-crystallization of iron oxides, formation of slag phase and secondary components. These processes take place at higher temperature. The firing temperature of the induration machine depends on chemical composition of the raw material, and flux addition. The optimum firing temperature of pellets within the induration furnace decides the pellet mineralogy, and pellet mineralogy in turn decides the physical and metallurgical properties of fired pellets. As for the firing of hematite pellets, more heat need to be supplied from external sources due to the absence of the exothermic reaction like that of magnetite oxidation. So the energy consumption of hematite pellet production is greater than that of magnetite pellets. In pelletization process, certain minimum temperature has to be attained in order to enable the necessary crystal transformations, and the reaction of oxide gangue constituents to generate the sufficient quantity of silicate/slag phase in hematite pellets to get optimum fired pellet properties. Moreover, the hematite pellet has poor roasting properties and do not achieve adequate strength until the roasting temperature is higher than 1300°C.1) Thus, it is necessary to maintain higher roasting temperature for hematite pellet as well as a narrower firing temperature range to get adequate bonding between the phases. In present pelletization process, to enhance the induration of hematite pellets, carbon burdened methods are found to be more favourable technique in practice. The JSW Steel Limited is a 10 mtpa integrated steel plant having 2×4.2 mtpa pellet plants and corex gas is used for firing of green pellets. To get the inherent fuel value of a hematite green ball equal to that of a magnetite pelletizing feed started to incorporate coke breeze in the pellet mix at JSW pellet plants. The firing temperature (from corex gas) of the induration machine and carbon addition in the pellet mix is interrelated to each other and decides physical and metallurgical properties of the pellets. At JSW Steel pellet plant the carbon addition varies from 0.90 to 1.50% and firing temperature varies from 1230 to 1320°C. Fluctuations in physical and metallurgical properties were observed due to deviation in carbon addition and firing temperature (TI – 90 to 95%, CCS – 180 to 230 kg/p, RDI – 10 to 18%, and reducibility – 0.56 to 0.60%). To get the desired physical and metallurgical properties of the fired pellets for iron making units the optimization of carbon addition with respect to firing temperature is required. Laboratory experiments have been conducted varying the carbon addition from 0.7 to 1.4% and firing profile from 1220 to 1330°C.
The production of iron ore pellets at JSW Steel Limited involves the drying of iron ore fines to get the moisture less than 1% and grinding the dried material to get the required fineness –45 μm size ≥62.0%. Prior to the formation of green pellets, the ground ore is mixed with small amounts of binding agents such as bentonite (0.7 to 0.9%), fluxes such as limestone to get the pellet basicity (CaO/SiO2) 0.40 to 0.50 and coke breeze as fuel (0.7 to 1.5%). The water is added to the mixed ore in the mixer to adjust the moisture content (0.8 to 0.9%). These give the pellets the proper physical and metallurgical properties needed in further processing.
The green pellets are formed in pelletizing discs and these pelletizing discs are connected to roller screens used for separating undersized (–6 mm) and oversized (+16 mm) pellets which are returned to the balling disc. The green pellets from the roller conveyor are evenly distributed (0.50 m height) across the width of the travelling grate induration furnace on hearth layer (0.05 m) for hardening of the pellets. The grate carries the green pellets through a furnace which consist of seven different zones i.e., updraft drying, downdraft drying, preheating, firing, after firing, cooling and after cooling zones. The induration furnace consists of three main steps 1. Drying of green pellets (200 to 350°C) 2 Firing of the pellets at 1250 to 1320°C to sinter the mix 3. Cooling of the hot pellets to (900 to 80°C) before discharging them on to the conveyors. In the induration furnace corex gas is used as fuel for firing purpose. Process air is circulated through these different zones with the help of five interconnected process fans. Figure 1 shows the process flow sheet of induration machine.
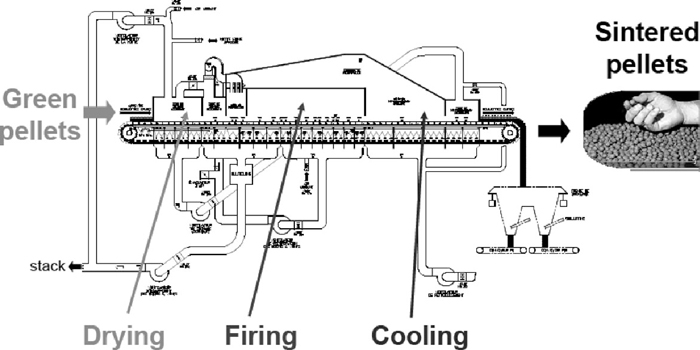
Schematic diagram of induration machine.
The burners in the preheat and firing zones are grouped into seven control zones. Each burner control zone has a thermocouple in the furnace hood which is connected via a temperature transmitter to the burner zone temperature controller. The temperature controller compares the temperature signal with the temperature set point and, accordingly regulates Corex gas flow control valve by means of current to pneumatic converter and a pneumatic operator. The burners operate as excess air burners with the furnace temperature controlled by adjusting the Corex gas flow to the burners and therefore the firing rate to maintain set point temperature.
Laboratory basket tests were carried out by varying the coke breeze addition from 0.70 to 1.40% in the green mix. The raw materials used for the preparation of the green pellets are iron ore, limestone, bentonite and coke breeze. The chemical analysis of the raw material is shown in the Table 1. Iron ore fines and limestone of –10 mm size and coke breeze of –6 mm size were ground separately in laboratory ball mill. Green pellet mixes for different level coke breeze were prepared by mixing the iron ore fines, limestone, and bentonite and coke breeze as per Table 2. Green pellets were prepared using laboratory scale balling disc. The green pellets were prepared in such a way that it should consist of ~97% –16+8 mm size range pellets. The details of balling disc are as follows:
| Description | % | |||
|---|---|---|---|---|
| Iron ore | Limestone | Bentonite | Coke breeze | |
| Fe (Tot) | 63.9 | 1.1 | 17.7 | 3.1 |
| SiO2 | 3.6 | 2.2 | 45.9 | 9.0 |
| Al2O3 | 2.3 | 0.5 | 16.1 | 5.0 |
| CaO | 0.1 | 51.8 | 2.6 | 2.0 |
| MgO | 0.0 | 1.4 | 2.3 | 0.3 |
| LOI | 2.2 | 42.0 | 8.7 | 5.0 |
| C | 79.7 | |||
| Mix 1 | Mix 2 | Mix 3 | Mix 4 | Mix 5 | Mix 6 | Mix 7 | Mix 8 | |
|---|---|---|---|---|---|---|---|---|
| Iron ore fines | 95.0 | 94.9 | 94.8 | 94.7 | 94.6 | 94.5 | 94.4 | 94.3 |
| Limestone | 3.5 | 3.5 | 3.5 | 3.5 | 3.5 | 3.5 | 3.5 | 3.5 |
| Bentonite | 0.8 | 0.8 | 0.8 | 0.8 | 0.8 | 0.8 | 0.8 | 0.8 |
| Coke breeze | 0.7 | 0.8 | 0.9 | 1.0 | 1.1 | 1.2 | 1.3 | 1.4 |
Disc diameter: 450 mm
Disc operating angle: 45°
Disc speed: 38 rpm
Each set of experiment comprises firing of 20 kg green pellets (40 numbers of experiments). The green pellets were kept in rectangular stainless steel baskets (500 mm long) and fired in pellet plant induration machine. The stainless steel basket was kept in the centre of the pellet bed on the hearth layer and it covered entire bed height of the induration machine. The corresponding operating parameters of the plant are shown in Table 3. In induration machine the firing temperature was varied from 1220 to 1340°C for each mix with different carbon content (0.70 to 1.40%). All experiments were carried out at the same machine parameters (Table 3). The firing temperature set points at different zones are shown in Fig. 2. Fired pellets were subjected to evaluations of chemical, physical and metallurgical properties.
| Description | |
|---|---|
| Feed rate, tph | 466 |
| Machine speed, m/min | 2.3 |
| bed height, mm | 500 |
| Hearth layer, mm | 50 |
| Drying, min | 15.2 |
| Preheating, firing and after firing, min | 18.7 |
| Cooling and after cooling, min | 15.8 |
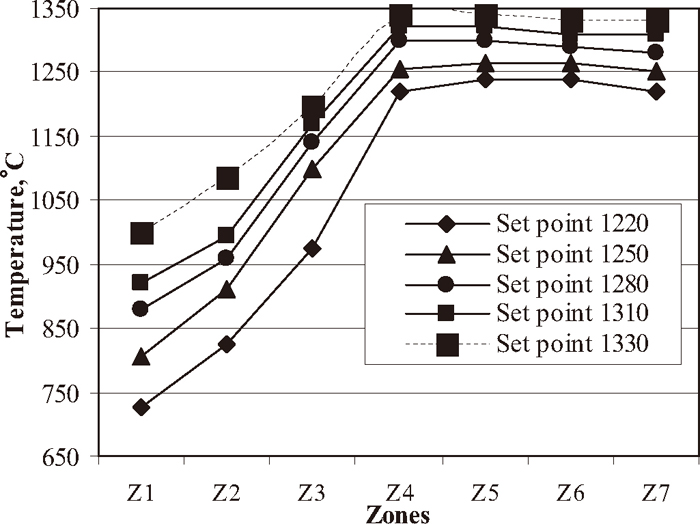
Firing profile at different temperature range.
Pellet samples of all experiments were collected for microstructural studies. For each study, the pellet sample was divided in to two pieces at the centre using the sample cutter. The one portion of the pellet sample was mounted in a sample holder using epoxy resin. These sections were polished using silicon carbide paper up to 1000 grit using water as a lubricant. A final polish was made by using diamond paste. For each experiment 2 samples were prepared for microstructural analysis.
Mineralogical characterization studies were carried out using optical microscope. The visible microstructural phases identified as hematite, magnetite, silicate/slag and pores based on the color appearance and shape.
Leica Q-win Image analyser software was used to provide an objective measurement of different phases in microstructure (hematite, magnetite, silicate and pore). The polished sample was placed under a microscope for examination. A camera is mounted behind the lens to capture the image. The eye piece of 50X objective lens was selected for the current study. Images of each sample were saved in the computer after capturing the image at different places through out the sample. For each sample 24 images were captured for phase analysis. To measure the area fraction of different phases in the pellet various tools of image analyser was used.
Figure 3 shows the influence of coke breeze addition on green pellet carbon content. With increase in coke breeze addition the over all green pellet carbon increased.
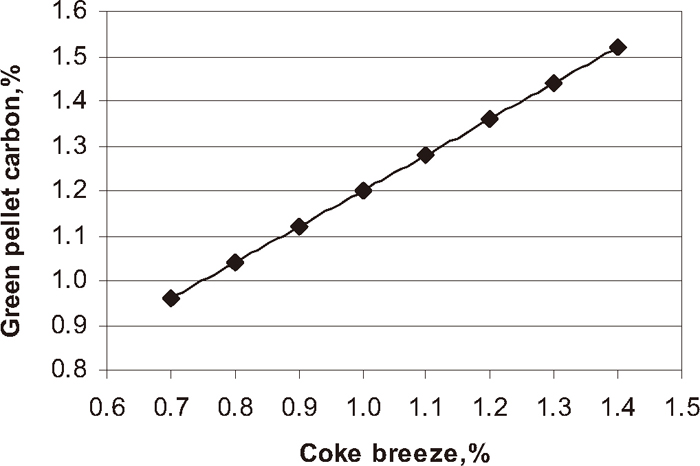
Influence of carbon coke breeze addition on total green pellet carbon.
Microstructural investigation was carried out by dividing the pellets into four segments as shown in Fig. 4. They are defined as:
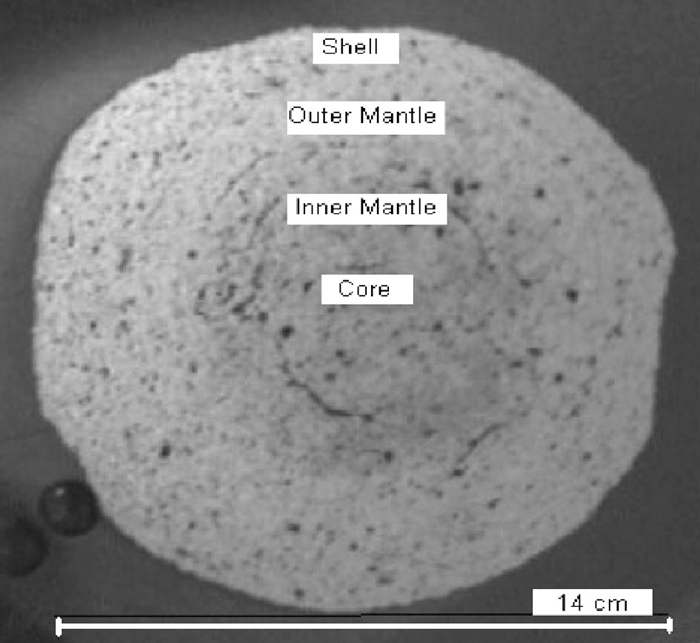
Segments of pellets.
(i) Shell: it extends from surface to 200 micron below
(ii) Outer mantle: just below the shell (2 mm thick)
(iii) Inner mantle: just below outer mantle (4 mm thick)
(iv) Core: innermost part of the pellet (4–5 mm in diameter).
Primary phases present in iron ore pellets are hematite (grey white) magnetite (grey white with pinkish), silicate/slag (dark grey), and pores (black).
The micrographs of pellet at optimum green pellet carbon content and firing temperature are shown in Figs. 5(a)–5(e). The micrographs from Figs. 5(a) to 5(e) at optimum green pellet carbon addition and firing temperature shows phase analysis with hematite, silicate and pore phase with minor quantity of magnetite phase. These micrographs consist of maximum hematite phase. We can observe re-crystallized hematite particles well bounded with silicate/slag phase. In hematite pellets balancing the slag formation and oxidation of magnetite phase is very important. These micrographs are associated with balanced slag phase with re-oxidised hematite crystals.
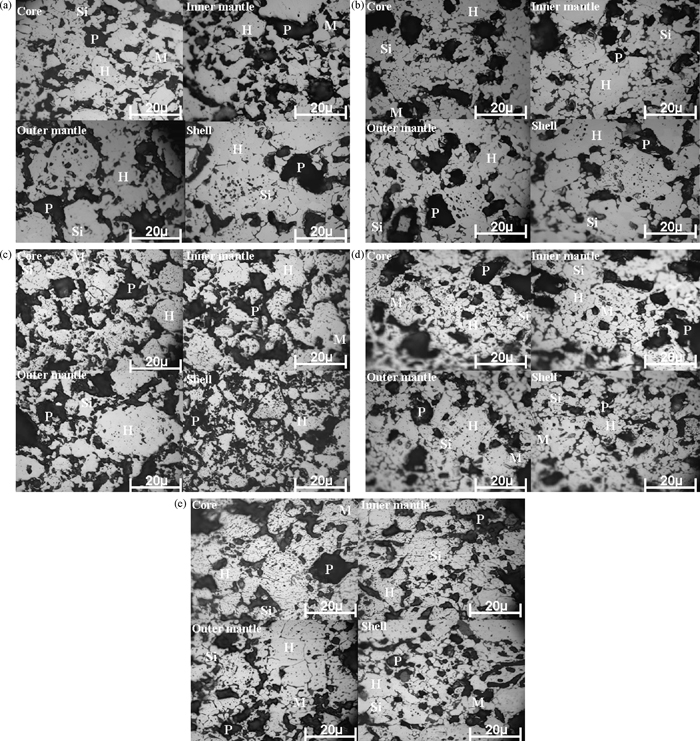
(a)–(e) Micrographs of pellet at optimum carbon and firing temperature.
The micrographs of pellets at higher green pellet carbon addition i.e. 1.52% at different firing temperatures are shown in Figs. 6(a)–6(e). At higher green pellet carbon addition at 1.52% the re-oxidation of magnetite phase was not completed. With increase in firing temperature with higher green pellet carbon content the magnetite phase increased from core to shell and we can observe the cracks at inner and outer mantle of the fired pellets. These pellets consist of large quantity of slag phase with magnetite phase. With increase in firing temperature with higher carbon addition the formation of long stretched pores are also increased. With increase firing temperature at higher green pellet carbon addition, the re-crystallization of hematite crystals were not observed in core and inner mantle.
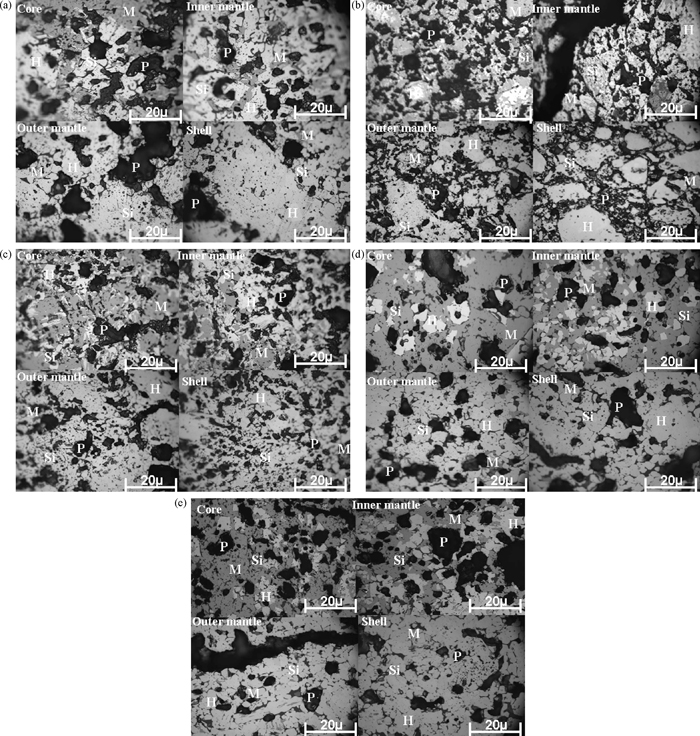
(a)–(e) Micrographs of pellet at higher carbon rate with different firing temperature.
Phase analysis was carried out on each experimental pellet to know the quantitative analysis of the fired pellets. The influence of green pellet carbon content on hematite, magnetite, silicate/slag and pore phase of the pellets at different firing temperatures are shown in Figs. 7, 8, 9, 10 respectively. The hematite phase decreased and magnetite phase increased with increase in green pellet carbon addition at different firing temperatures. As temperature increases from 1220 to 1330°C the hematite content decreased and magnetite content increased. Lower hematite and higher magnetite phase at the firing temperature 1330°C was observed. The silicate phase increased and pore phase decreased with increase in green pellet carbon addition. As temperature increases from 1220 to 1330°C the silicate phase increased and pore phase decreased. We observed higher silicate phase and lower pore phase at firing temperature 1330°C.
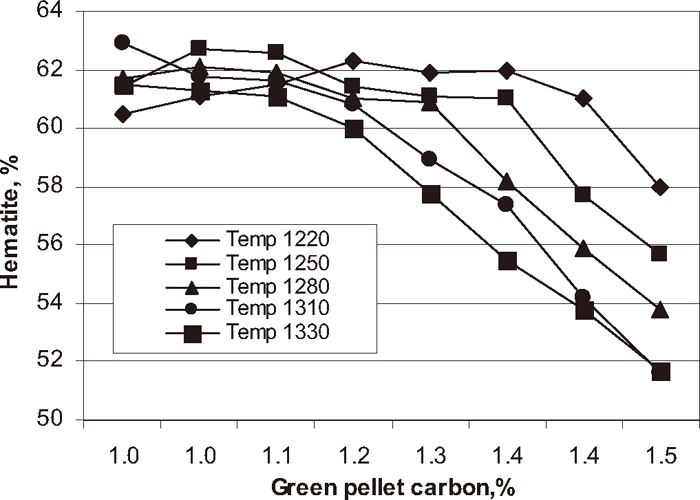
Influence of carbon addition on hematite phase at different temperature.
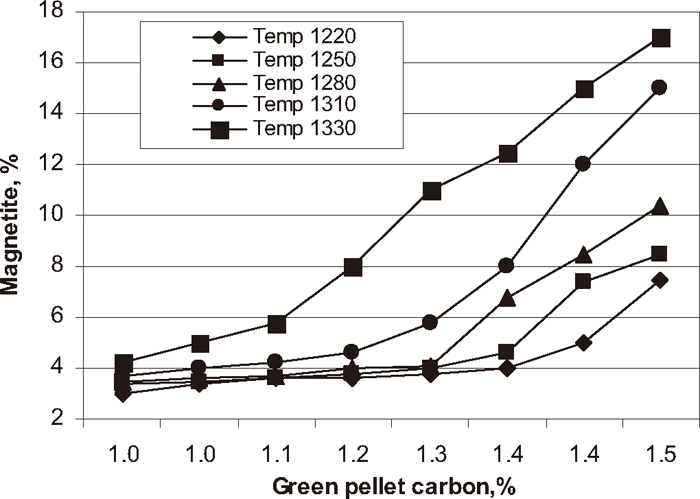
Influence of carbon addition on magnetite phase at different temperature.
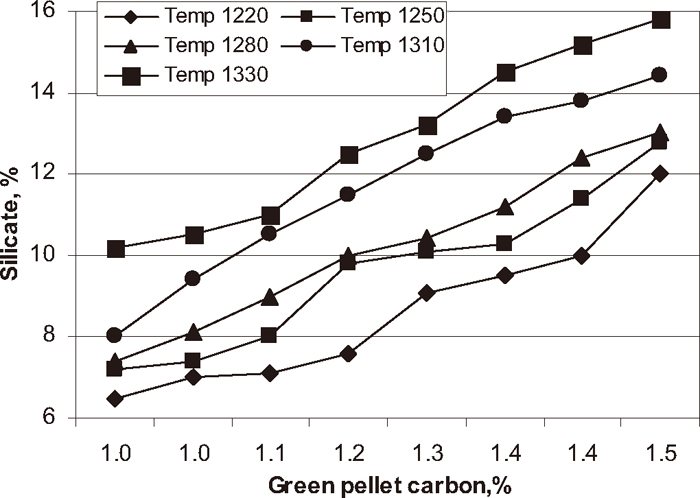
Influence of carbon addition on silicate phase at different temperature.
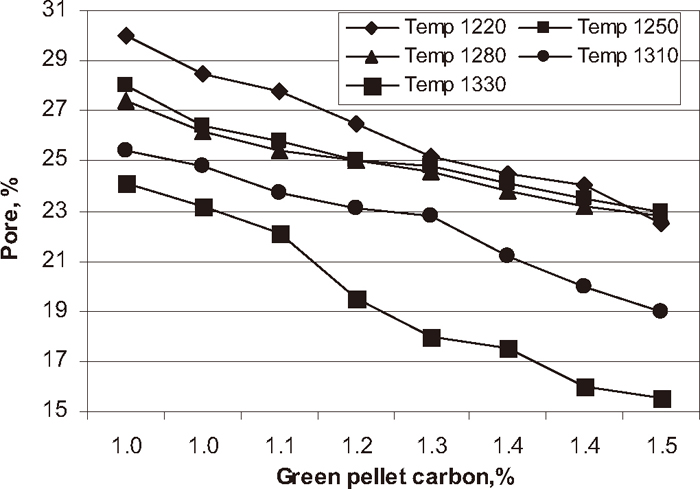
Influence of carbon addition on pore phase at different temperature.
In pellet plant, to get the required chemistry, physical and metallurgical properties of the pellets for iron making units, a proper firing profile must be established to balance the internal heat by solid fuel and external heat by gas or oil. At JSW Steel Limited Corex gas is used as external fuel to fire the iron ore green pellets. As per Fig. 3 the total green pellets carbon content of the pellets increased with increase in coke breeze addition. Figure 11 shows the influence of green pellet carbon content on FeO content of the fired pellet. The FeO content of the fired pellet increased with increase in green pellet carbon addition at different firing temperature from 1220 to 1330°C. The Fig. 12 shows the influence of magnetite phase on FeO content of the pellets at different temperature from 1220 to 1330°C. The FeO content of the pellet increased with increase in magnetite phase. In general as higher the amount of solid fuel higher is the formation of magnetite phase. Depending on the pellet carbon content and firing conditions, secondary magnetite may or may not be completely re-oxidised to hematite after cooling.2) The FeO content of the pellets are directly proportional to magnetite content. Higher the amount of magnetite phase in the fired pellets the higher amount of FeO content. The formation amount of magnetite phase increases with increasing the firing temperature and/or with increasing the carbon content. The oxidation of magnetite phase to hematite phase is very difficult to occur under the condition where the formation of magnetite phase proceeds. Higher coke addition at higher firing temperature may even results in a further reduction towards the formation of wustite, which, within the time available, does not re-oxidise and form fayalite with the presence of silica.
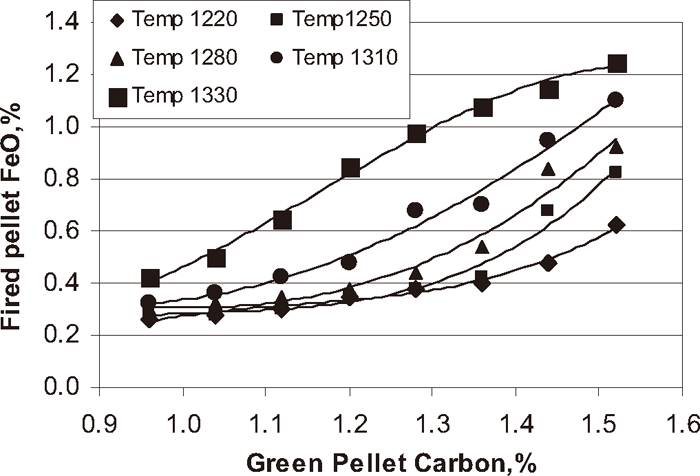
Influence of green pellet carbon addition on fired pellets FeO content.
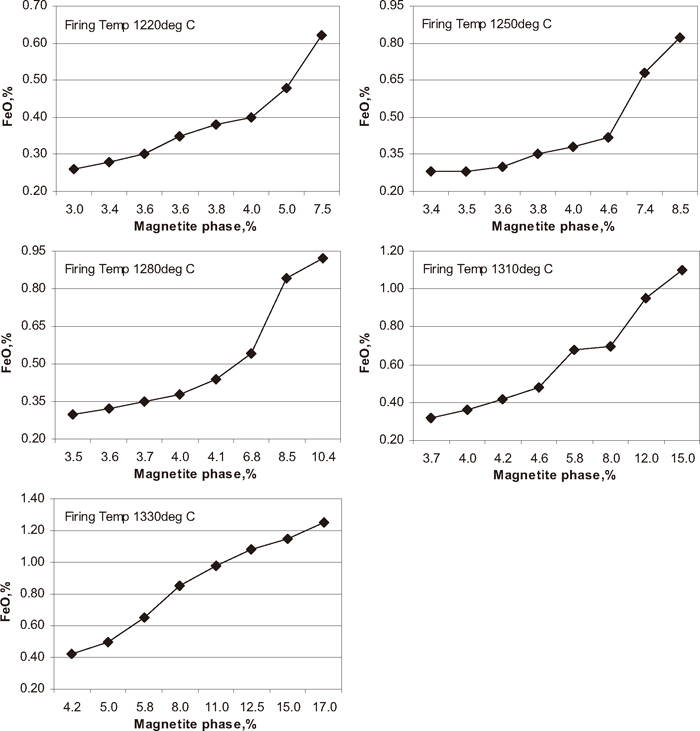
Influence of magnetite phase percentage on FeO content of fired pellets.
Tumbler index (T.I), is the measure of resistance to generate fines during handling and transportation. Figure 13 shows the influence of green pellet carbon content at different temperature on tumbler index of the pellets. With increase in green pellet carbon content of the pellet at the different temperature 1220, 1250, 1280, 1310, and 1330°C the tumbler index of the pellet increased up to optimum carbon addition 1.3, 1.2, 1.0, 0.9, and 0.7% respectively and after that decreased with increase in carbon addition.
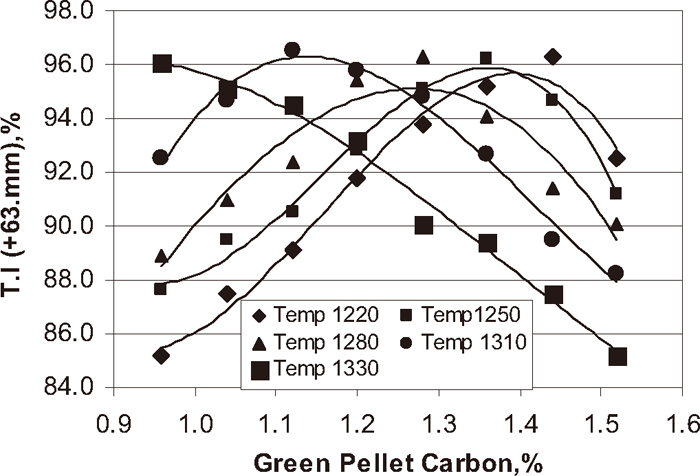
Influence of green pellet carbon content on T.I of the pellets.
CCS of the pellets plays a significant role in the performance of Corex and Blast furnace processes. Pellets with low strength cannot withstand the handling loads during their shipping and load of burden in the reduction furnace. Figure 14 shows the influence of green pellet carbon content at different temperature on cold crushing strength of the pellets. With increase in green pellet carbon content of the pellet at the different temperature 1220, 1250, 1280, 1310, and 1330°C the CCS of the pellet increased up to optimum carbon addition 1.3, 1.2, 1.0, 0.9, and 0.7% respectively and after that decreased with increase carbon addition. The tumbler index and CCS of the pellets are influenced by FeO content of the pellet. Figure 15 shows the influence of FeO content of the pellet on T.I and CCS of the pellet. With increase in FeO content of the pellet at different firing temperatures T.I and CCS of the pellet increased up to optimum FeO content 0.40 to 0.50% and after wards decreased with increase in FeO content of the pellet. At lower carbon addition, the FeO content may be lower than 0.5% and it is even good for pellet strength but at lower FeO content of fired pellet below 0.40% pellet strength was poor may be due to insufficient bonding of the hematite particles due to poor firing. For FeO content of the fired pellet above 0.50% the pellet T.I and CCS was poor due to magnetite content, more porosity, long stretched pores and generation of stress inside the pellet. Conversion of magnetite to hematite is a strongly exothermic reaction and favours the grain growth and sintering of the particles of iron ore concentrate to form hard, strong pellets.3) As the coke breeze increases this effect becomes more and more severe. During firing the carbon monoxide is generated in pellet due to incomplete combustion of coke breeze. This carbon monoxide reduces the hematite to magnetite. As a result, a duplex structure is formed across the cross section of the pellet with hematite predominantly in the outer mantle and shell and magnetite in the inner mantle and core (Figs. 6(d) and 6(e)). Bond and lattice strains caused due to the differential contraction/shrinkage of these phases during cooling causes cracks and these cracks reduce the pellet strength. The higher FeO pellets are associated with duplex structure i.e. magnetite core and hematite shell attributed to poor strength (Figs. 6(d) and 6(e)). The optimum FeO content of the pellet should be 0.40 to 0.50% to achieve better physical properties of the pellets. To avoid the formation of magnetite at higher coke rate & higher temperature and to avoid poor firing of the pellets at lower temperature & lower carbon addition the addition of coke breeze as carbon source and firing temperature of the pellet should be properly controlled.
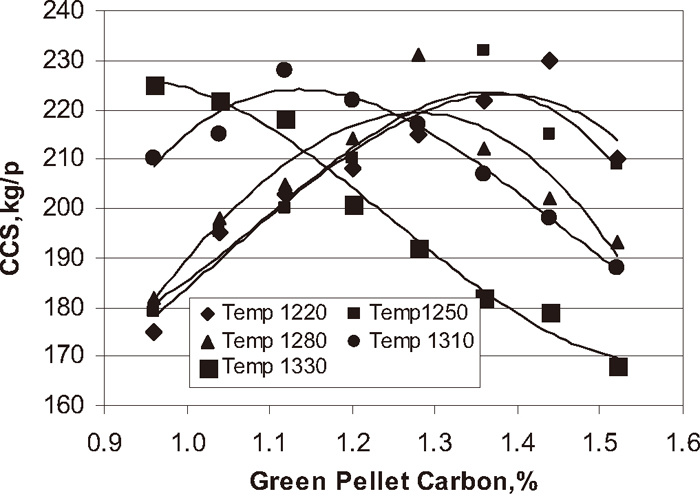
Influence of green pellet carbon content on strength (CCS) of the pellet.
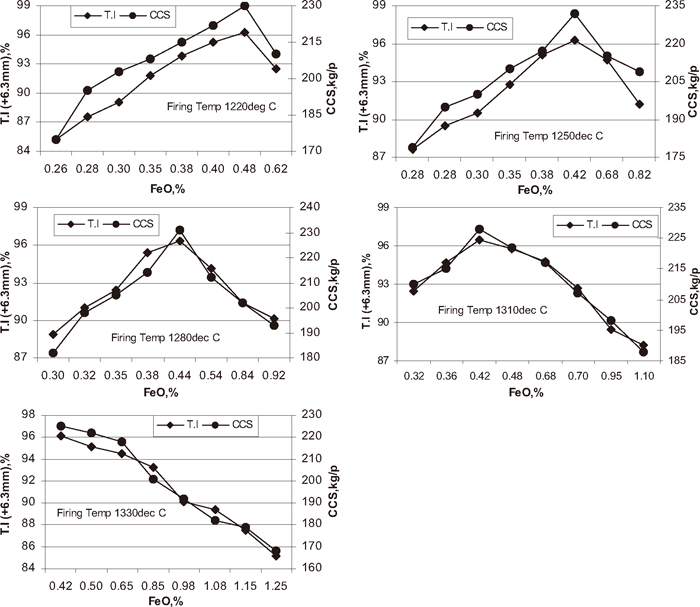
Influence of FeO content on pellet strength (T.I).
The reduction degradation index of pellet is defined as a quantitative measure of the degree of disintegration, which could occur in the pellet in the upper part of the blast furnace after some reduction at lower temperature. The primary cause of disintegration at lower temperature is thought to be the crystalline transformation during the formation of cubic magnetite from hexagonal hematite generating stresses, which leads to the weakening of pellets. It is very clear that iron oxide in the indurated pellet is mainly in the form of hematite; therefore, generation of internal stress is unavoidable. As the coke breeze content increases with firing temperature the effect becomes more and more severe. Figure 16 shows the influence of coke breeze addition at different temperature on reduction degradation index of the pellet. With the addition of coke breeze at different temperature the reduction degradation index of the pellet decreased up to optimum coke breeze addition and firing temperature and again increased with increase in carbon addition. At higher green pellet carbon addition 1.52% and at higher temperatures higher quantity of slag and magnetite phases were formed. Along with the magnetite phase we can see the number of cracks formed due to the generation of stress inside the pellet and this leads to poor strength. Poor strength pellets cannot sustain mechanical loading during reduction and hence adversely affects the RDI.4) Higher the magnetite phase, higher the FeO and lower the pellet RDI. Pellets with higher carbon content are associated with higher magnetite/FeO and silicate phase and the pellet RDI was at higher side due to the internal stress created during the crystal transformation. At lower carbon addition and lower firing temperature the RDI of the pellet was higher side due to formation of less slag phase and poor bonding of slag phase with hematite particles. The pellet porosity also decides the RDI of the pellet. The volume increase at the hematite to magnetite transition generally fluctuates between 10 to 15% instead of 5% excepted in literature.5) This apparent swelling appears along with the development of inner grain porosity as long stretched pores (Figs. 6(a)–6(e)). The higher the quantity of green pellet carbon addition (1.52%) with increase in firing temperature formation of long stretched pores increased and deteriorated the sinter strength and RDI. The optimum RDI required for iron making units are RDI (–6.3 mm) should be less than 11.5%. To get the optimum pellet mineralogy balancing of pellet porosity, slag formation and oxidation of magnetite is necessary. The balanced mineralogy can be achieved by balancing the carbon addition with respect to firing temperature. At firing temperature 1220, 1250, 1280, 1310 and 1330°C the optimum carbon addition of 1.30, 1.20, 1.10, 0.90 and 0.70% achieved the pellet RDI ≤11.5% for iron making units respectively.
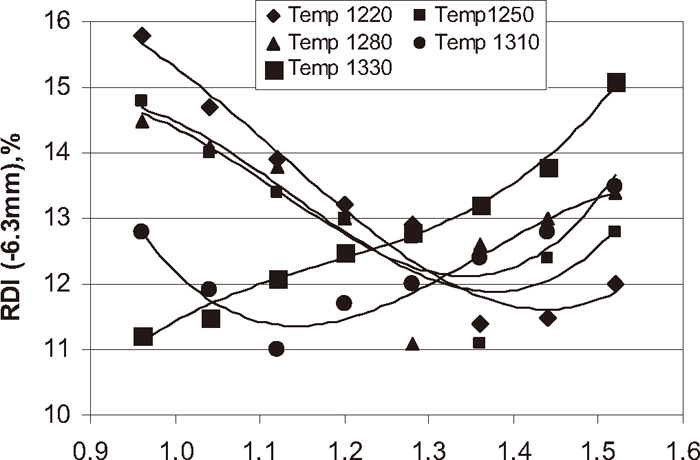
Influence of green pellet carbon content on pellet RDI.
Reducibility is the ease with which the oxygen combined with the iron can be removed. The reducibility is generally compared at 40% degree of reduction. The influence of green pellet carbon content on pellet reducibility is shown in Fig. 17. Reducibility of iron ore pellet decreased with increase in green pellet carbon addition at different temperatures. The pellet fired at 1220°C shows higher reducibility and pellet fired at 1330°C shows lower reducibility with increase in carbon addition. Reducibity of the pellet mainly depends on the magnetite content of the pellet and porosity. Figure 18 shows the influence of magnetite phase on pellet reducibility. The pellet reducibility decreased with increase in magnetite phase percentage at different firing temperature. In iron ore pellet hematite is much more reducible than magnetite. It is highly desirable that the all the lower oxides (magnetite/FeO) of the pellet formed during heating should be oxidized to hematite during cooling cycle. Higher magnetite content in the pellet decreases the pellet reducibility.

Influence of green pellet carbon content on pellet reducibility.
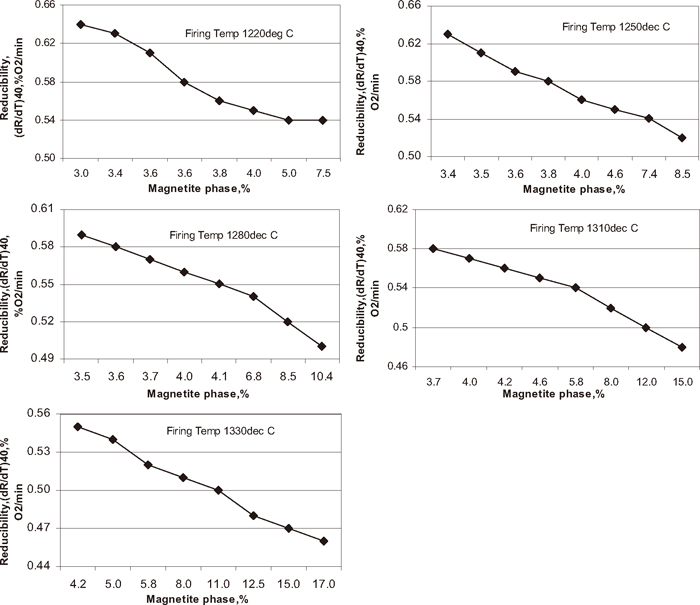
Influence of magnetite phase on pellet reducibility.
Pores also influence the properties of iron ore pellet. Pores provide diffusion path for reactant gases and surface area for reaction. Figure 19 shows the influence of pellet porosity on pellet reducibility. With increase in pore phase pellet reducibility increased. With increase in firing temperature the pellet porosity decreased and pellet reducibility also decreased. The decrease in pellet porosity is due to increase in silicate/slag phase of the pellet due to increase in carbon addition and firing temperature. Many researches found that reducibility was increased with increasing porosity and strength was increased with decreasing porosity.6,7)
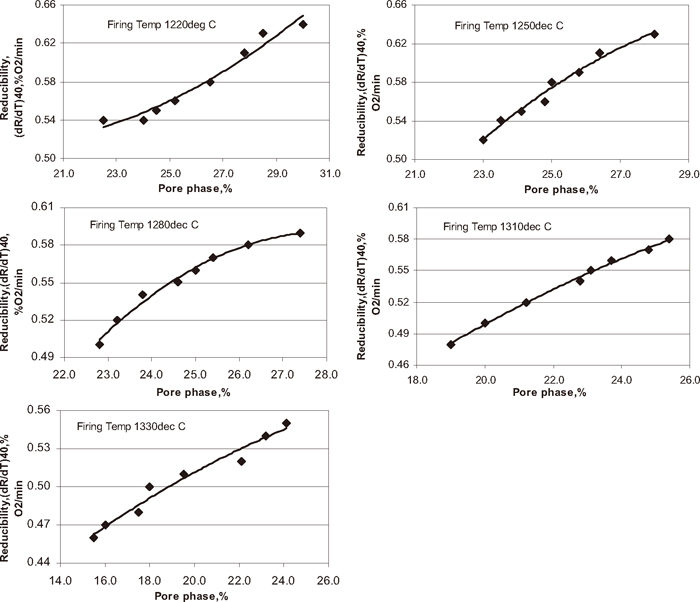
Influence of pore phase on pellet reducibility.
In a single homogeneous pellet, the microstructure after induration depends mainly on the bed permeability, carbon content, temperature cycle and O2 pressure in the same area. The process of complete oxidation of reduced phases is dependent on exposure of these phases to oxygen. The overall oxygen partial pressure was partially controlled by the carbon rate. However, higher addition of the carbon beyond a certain limit and particular firing temperature badly impairs the pellet quality. The optimum coke breeze addition and firing temperature is an effective measure to promote oxidation. The higher content of carbon & higher firing temperature of the pellets decreases oxidation of magnetite into hematite and pellet remains with magnetite, and slag phase.
Green pellet carbon addition increased with increase in coke breeze addition.
(1) The mineralogical phases like hematite & pore phase decreased and magnetite and slag phase increased with increase in coke breeze addition. At highest firing temperature pellet showed lower hematite & pore phase and higher magnetite and slag phase.
(2) The FeO content of the fired pellet increased with increase in green pellet carbon addition at different firing temperature from 1220 to 1330°C due to increase in magnetite phase.
(3) With increase in FeO content of the pellet at different firing temperatures T.I and CCS of the pellet increased up to optimum carbon content 0.40 to 0.50% and after wards decreased with increase in FeO content of the pellet.
(4) With increase in green pellet carbon content at different temperature 1220, 1250, 1280, 1310, and 1330°C the tumbler index and CCS of the pellet increased up to optimum carbon addition 1.3, 1.2, 1.0, 0.9, and 0.7% respectively and after that decreased with increase in carbon addition. The tumbler index and CCS of the pellets are influenced by FeO content of the pellet.
(5) With the addition of coke breeze at different firing temperature (1220, 1250, 1280, 1300, and 1330) the reduction degradation index of the pellet decreased up to optimum coke breeze addition (1.3, 1.2, 1.1, 0.9, and 0.7) and firing temperature and again increased with increase in carbon addition. Pellets with higher carbon content and firing temperature are associated with higher magnetite/FeO and silicate phase even though, the pellet RDI was at higher side due to the internal stress created during the crystal transformation. At lower carbon addition and at lower firing temperature the RDI of the pellet was higher side due to formation of less slag phase and poor bonding of slag phase with hematite particles.
(6) Reducibility of iron ore pellet decreased with increase in green pellet carbon addition at different temperatures. The pellet fired at 1220°C shows the higher reducibility due to lesser magnetite, slag & higher pore phase and pellet fired at 1330°C shows the lower reducibility due to higher magnetite, slag phase & lower pore phase with increase in carbon addition.
(7) At firing temperature 1220, 1250, 1280, 1310 and 1330°C the optimum carbon addition 1.30, 1.20, 1.10, 0.90 and 0.70% achieved the optimum physical and metallurgical properties of the pellet for iron making units respectively.