2014 Volume 54 Issue 1 Pages 131-135
2014 Volume 54 Issue 1 Pages 131-135
Various investigations were conducted to establish quantification of microelements in iron and steel using ICP–MS with solid-phase extractive separation capable of separating the matrix simply and rapidly as a pretreatment. A “zero-emission type analysis” free from EDTA as a masking agent and buffering agents used for pH adjustment was sought. To do so, an anion-exchange type solid-phase extraction disk was used as the solid-phase extraction agent, along with “skill-free analysis” requiring no cumbersome manipulations, and “quick analysis” for assessment in an extremely short period of time. Results show a target element in a solid-phase disk, whereas the sample solution at separation was maintained at higher than pH 1.8. The pH adjustment was conducted by dilution of the sample solution with about 400 cm3 of water. The target element held in the solid-phase disk was eluted with 10 cm3 of 3 kmol/m3 nitric acid. The detection limitations [3 σ; ng/g (ppb)] were the following: Ti 0.043, Ge 0.014, Zr 0.013, Nb 0.025, Mo 1.06, Sn 0.030, Hf 0.010, Ta 0.019, and W 0.12.
Regarding studies of high-purity iron and steel, ever higher sensitivity and higher accuracy have been required.1) Many researchers are seeking analytical methods for use with ultra-trace elements.2,3) At present, inductively coupled plasma mass spectrometry (ICP–MS) is used widely for quantification of trace impurities in iron and steel. However, reduced sensitivity arises from matrix interference and other causes when a sample solution is introduced directly into the apparatus after acid decomposition. Therefore, separation of iron in the matrix is necessary. To date, coprecipitated separation using cupferron for separation of the iron,4) solvent extractive separation for removal of the iron using organic solvent,5,6,7) and solvent extractive separation for extraction of a target element as a metallic chelate using chelate reagent8,9) have been used in addition to other methods. However, organic solvents that are detrimental to the environment and human health are used for solvent extractive separation. Moreover, these operations involve cumbersome separation manipulations. In recent years, “zero-emission type” analysis, which uses no detrimental reagents at all or reduces their amount to the greatest degree possible, skill-free analysis, which everyone can accomplish with ease without depending on complicated techniques or technology, and rapid analysis have been attracting attention. Reduction to environmental load was demanded in the field of steel analysis, and those studies were performed in the meeting for the study of the development of environment-friendly steel analysis techniques10) in ISIJ. Under such circumstances, ion-exchange separation,11,12,13,14) which meets these conditions, has been studied extensively. However, considerable time is necessary because this method uses no absorption filtration with a large quantity of ion-exchange resin when a sample solution is passed to a column. In addition, time is necessary for conditioning and reprocessing of the resin. The author investigated extremely simple separation methods without using detrimental organic solvents in the microelement quantification method in high-purity iron and steels. These methods include one15) that uses chromazurol B, known as a complex forming agent of iron, as the collecting agent of iron, in addition to solid-phase extraction16,17) using chemical bonding type silica gel as the solid-phase extraction agent. With this solid phase extraction for separation of iron, the sample solution is passed through a solid-phase column in which 0.5 g of chemical bonding type silica gel is filled into a 15 cm3 syringe. Suction filtration is used. Therefore, treatment can be completed in a shorter period of time. In addition to the iron matrix, favorable results have been obtained for the separation of aluminum,18) molybdenum,19) and tungsten.20) In this study, solid-phase extraction giving consideration to the environment is also applied as the separation method similarly to that reported previously to achieve added rapidity and simplicity of manipulations.
The solid-phase extraction agent used for this study is a disk-type solid-phase extraction agent (47-mm-diameter, 0.5 mm thickness) that brought good results, as reported previously.21,22) In this study, instead of a cation-exchange type solid-phase disk reported previously, an anion-exchange type solid-phase extraction disk having a disk profile in which anion-exchange type resin is hooked by ultrafine fibers made of PTFE is used. The base material of the anion-exchange resin is quaternary ammonium. This material provides such advantages that, as compared with chemical bonding type silica gel as reported previously, conditioning can be performed simply and large amounts of the sample solution can be treated rapidly. Although ethylenediaminetetraacetic acid (EDTA) was used for masking of iron in the solid-phase extraction in a previous study, we sought to establish an improved “zero-emission type” analysis method in this study. In other words, hydrofluoric acid was used for sample decomposition, devoting attention to PTFE of the base material, which is another advantage of using a disk type solid-phase agent. Then, the method of separating iron as a fluoride was examined.
Results show that operability and analysis time were greatly improved in comparison with the conventional ion exchange separation method.
Both ICP–MS (ELAN 6000; PerkinElmer Inc., USA) and ICP–AES analyses (OPTIMA 3300DV; PerkinElmer Inc., USA) were performed. Conditions for separation were analyzed and residual iron in the measured solutions was quantified. A vacuum filter holder “47 mm polysulfone holder” equipped AS-75 Aspirator (Advantec MFS Inc., Japan) was used to perform solid extraction.
2.2. ReagentsAn iron solution (concentration: 50 kg/m3 (50 mg/ml), 25 g high-purity iron (JSS 001-4 or JSS 001-5: Certified Reference Material of the Japan Iron and Steel Federation) was placed in a 500 cm3 beaker and decomposed using 25 cm3 of nitric acid. After cooling, the solution was moved to a 500 cm3 volumetric flask and diluted with water to a constant volume. The Standard Solution for Atomic Absorption (Cica-Merck, Japan) (Ti, V, Ge, As, Zr, Mo, Sn, Ba, Ce, Hf, W, Ta, Sb and Nb: 1 kg/m3 (1 mg/ml) for each element) was also used. Each element was mixed and diluted to 0.001 kg/m3 (14-element mixed solution). A standard solution (Ti, Ge, Zr, Mo, Sn, Hf, W, Ta and Nb: each 1 kg/m3 of element) for atomic absorption in the calibration curve of the real sample made in Kanto Chemical was mixed and diluted. It was adjusted and used (nine-element mixed solution) for the density of each 0.001 kg/m3 of elements. Residual iron measured in the solution was quantified using the iron solution 1 kg/m3: The Standard Solution for Atomic Absorption (Cica-Merck, Japan). Empore™ extraction disks “anion-SR” (3M Co., USA) were used as the solid phase extraction chemical. Anion-SR is a poly (styrenedivinylbenzene) copolymer that has been modified with quaternary ammonium groups. Functional group quaternary ammonium controlling ion exchange has been introduced into a part of the benzene ring of the ion-exchange resin frame. Showed the structural formula in Fig. 1.
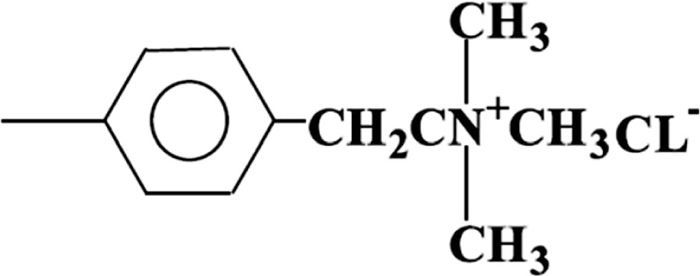
Structural formula of quaternary ammonium.
The reagents used for the conditioning extraction disks were methanol of super-high grade reagent (Cica-Merck, Japan). Other reagents were hydrofluoric acid (38 mass%), nitric acid (68 mass%), hydrochloric acid (30 mass%) and hydrogen peroxide (35 mass%) (Tamapure-AA100 Ultra pure Analytical Reagent; Tama Chemicals, Japan). The water was refined using an apparatus for producing ultrapure water (Milli-Q SP TOC; Millipore Corp., USA); it was refined further using a distilled water production apparatus with a non-boiling technique (Fujiwara Scientific Co., Ltd., Japan).
2.3. Experimental ProceduresThe iron solution (2 cm3, 50 kg/m3, nitric acid acidity) prepared in 2.2 was transferred to a 500 cm3 polypropylene beaker. Then 1 cm3 of hydrofluoric acid, 2 cm3 of hydrogen peroxide solution, and a constant amount of a 14-element mixed solution were added and diluted with water. This solution was passed through the solid-phase disk. Then the target element was held alone in the solid-phase disk to separate it from iron. After washing with water, it was eluted from the solid-phase disk using 3 kmol/m3 nitric acid or 3 kmol/m3 hydrochloric acid. It was then brought to a constant volume of 100 cm3. Measuring conditions are presented in Table 1.
| ICP-AES conditions | |
| RF Power | 1.3 kW |
| Plasma gas (Ar) | 15.0 l min–1 |
| Auxiliary gas (Ar) | 0.5 l min–1 |
| Carrier gas (Ar) | 0.8 l min–1 |
| Wavelength (nm) | Fe: 239.562, |
| ICP-MS conditions | |
| RF Power | 1.0 kW |
| Plasma gas (Ar) | 15.0 l min–1 |
| Auxiliary gas (Ar) | 1.2 l min–1 |
| Carrier gas (Ar) | 0.68 l min–1 |
| Mass | Ti(48) V(51) Ge(74) As(75) Zr(90) Nb(93) Mo(98) Cd(111) Sn(120) Sb(121) Ba(138) Ce(140) Hf(180) Ta(181) W(208) |
The solution used for addition and collection experiments of the target element was prepared such that 10 cm3 of the 14-element mixed solution (0.001 kg/m3) was added to the blank solution obtained through all manipulations, transferred to a 100 cm3 measuring flask, and brought to a constant volume.
For conditioning of the solid-phase disk, 10 cm3 of methyl alcohol is dropped on the disk and left for 30 s. Next, 10 cm3 of water and 10 cm3 of 3 kmol/m3 nitric acid (3 kmol/m3 hydrochloric acid for the case eluent is hydrochloric acid) were introduced into series and rinsed finally with 30 cm3 of water.
2.4. Recommended ProcedureBefore heating and decomposition of the 0.100 g sample, 1 cm3 hydrofluoric acid, 1 cm3 nitric acid (1+1) and 2 cm3 of hydrogen peroxide were added to a 100 cm3 beaker (PTFE). After cooling, the solution moved to a 500 cm3 polypropylene beaker, the pH was adjusted 1.8 or more with the water used for dilution. This solution was passed through the solid-phase disk that had been conditioned to retain the target elements in the extraction chemicals. The solid-phase extraction disc was rinsed several times and the rinse water was discarded. The eluent, 10 cm3 of 3 kmol/m3 nitric acid, was then passed through the solid-phase extraction disk to separate the target elements. After several rinses with water, the eluate and rinse water were moved to a volumetric flask to maintain a constant volume of 100 cm3. The solution introduced to ICP–MS was measured to assess conditions shown in Table 1. Concentrations of the target elements were calculated using a calibration curve. The analytical method described above applies to the determination of the four elements of Nb, Zr, Ti, and the Ta. The calibration curve of five elements of Hf, W, Sn, Ge and Mo is different from four elements of the mentions above. Specifically, these five elements are not contained or contents of known high-purity iron are prepared, with decomposition of the iron according to the analytical method described above. Nine element mixtures (0.001 kg/m3) are added to this solution progressively. Then the processing is performed as that for the sample and production of the calibration solution. Similarly, the specified element density is measured and calculated from a calibration curve.
Iron became a cation. It was not retained in the solid-phase extraction chemicals of the anion exchange because the sample was decomposed with hydrofluoric acid and nitric acid and then added to a hydrogen peroxide solution. In contrast, because impurities Nb, Mo, W, Hf, etc. in the sample were oxoanions, they were retained with the extraction chemicals. They were separated under optimum conditions using a 14-element mixed solution. Then they were measured using ICP–MS. Among the 14 target elements of Ti, V, Ge, As, Zr, Mo, Sn, Ba, Ce, Hf, W, Ta, Sb, and Nb, recovery rates higher than 50% were obtained from 9 of them (Ti, Ge, Zr, Mo, Sn, Hf, W, Ta, and Nb). Optimum conditions to separate the nine target elements are described below.
To determine the optimum separation conditions, the optimum pH region at the iron matrix separation was checked. According to the manipulation described in 2.3, 1 cm3 of hydrofluoric acid, 2 cm3 of hydrogen peroxide solution and a 9-element mixed solution were added to the iron solution. In this manner, five solutions with this composition were prepared. For pH adjustment of the sample solution, buffering solution or the like was not used at all because of the desire for zero emissions. The pH of each solution was changed stepwise from 1.2 to 2.0 by changing the amount of added water used for dilution. For example, the total amount of the solution for the pH 1.2 case is about 100 cm3, and about 400 cm3 for the pH 1.8 case. These solutions were passed through the solid-phase disk. The target element was adsorbed to the solid-phase extraction agent. For elution, 10 cm3 of 3 kmol/m3 nitric acid was used and brought to a constant volume of 100 cm3. This solution and the solution for addition and collection experiments in 2.3 were measured using ICP–MS. The collection rate of the target element was checked at each pH. The results are presented in Fig. 2. The collection rate of Nb was 100% at pH 1.4–2.0, whereas those of Ti, Ta, and Zr reached nearly 100% at greater than pH 1.8. Those of W, Hf, Sn, Ge, and Mo showed a uniform increase in the collection rate as pH increased from about 50% to 80%. Other elements were undetected in all pH areas as well. Referring to those results, optimum pH at separation is apparently higher than pH 1.8. Moreover, separation should be performed after dilution with more than about 400 cm3 of water. This pH adjustment is greatly dependent on the pH after water is used for dilution. Therefore, it is necessary to ascertain in advance the amount of water necessary for adjustment to pH 1.8 or higher pH. Residual iron in these solutions was quantified in each pH area using ICP–AES. The results indicate that iron of approximately less than 0.002 kg/m3 was observed in all areas, suggesting that this amount would have no effect on ICP–MS measurement.

Effect of pH on the recovery of analytes. Fe: 1.00×10–4 kg, Analyte: 1.00×10–10 kg, Eluent: 3 kmol/m3 HNO3 10 cm3.
The author investigated the eluent for eluting the target element held in the solid-phase agent. Although nitric acid is normally used as the eluent considering the influences on ICP–MS, hydrochloric acid was included in the investigation target because Sn and others are included in the analysis target element. The hydrochloric acid is used as eluate of the Sn, but is usually excluded from the choice because a compound of chlorine affects the ICP–MS measurement. Effects on ICP–MS measurements, optimum concentration, and the amount used were checked. As described in 3.1, a nine-element mixed solution was added to the iron solution transferred to 500 cm3 beaker, and water was added to adjust it to higher than pH 1.8. Thereby, ten solutions with this composition were prepared. These solutions were passed through the solid-phase extraction disk. The target element was held in the solid-phase. After washing several times with water, 2.5, 5.0, 7.5, 10.0, and 12.5 cm3 of 3.0 kmol/m3 nitric acid solution or hydrochloric acid solution was passed through it to elute the target element. After washing several times with water, each eluate and each wash liquid were transferred to a 100 cm3 measuring flask to obtain constant volume. At the same time, a solution was prepared similarly to 3.1 by adding a nine-element mixed solution to the blank solution. These solutions were measured using ICP–MS to assess the relation between the eluent concentration and its collection rate. Figure 3 presents the results. When 3.0 kmol/m3 nitric acid was used for eluate, for the four elements of Nb, Zr, Ti and Ta, 90–110% recoveries were provided in a concentration of 7.5–12.5 cm3 range. The Hf, W, and the Sn were 70–80% of recoveries in all density ranges. Regarding the Ge and the Mo, approximately 50–60% of recoveries were provided in a concentration of 7.5–12.5 cm3 range. In addition, when 3.0 kmol/m3 hydrochloric acid was used for eluate, only Ta got 100% of recoveries, but was not able to obtain an expected result for other elements. The remarkable difference of the nitric acid eluate and hydrochloric acid eluate was recovery of the Mo: when hydrochloric acid eluate was used, the recovery was less than 20%, which suggests that a chlorine compound affects the ICP–MS measurement in the Mo. Consequently, 3.0 kmol/m3 nitric acid of 10 cm3 was selected as the eluate. For the five elements of W, Hf, Sn, Ge, and Mo, collection rates were 50–80%. Therefore, quantification is possible if a calibration line using the same manipulations as those used for the target element quantification is used. The calibration is done as shown in a sample of about five elements of Hf, W, Sn, Ge, and the Mo. The iron concentration remains increased if more than 10 cm3 of 3.0 kmol/m3 nitric acid is used. However, because it is less than 0.002 kg/m3, even if as much as 20 cm3 nitric acid is used, we infer that measurements using ICP–MS, the apparatus body, detectors, etc. are unaffected.
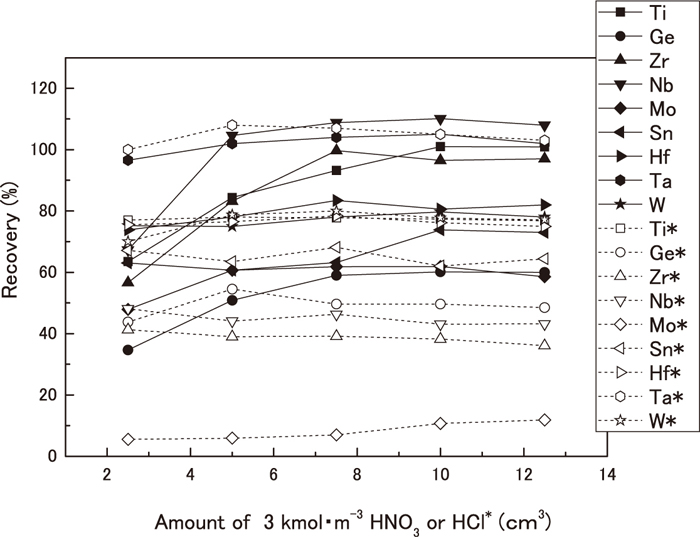
Effect of eluate concentration on the recovery of analytes. Fe: 1.00×10–4 kg, Analyte: 1.00×10–10 kg, pH: 1.8.
The nine-element mixed solution (10 cm3) was added to the blank solution and solid-phase extraction was performed. The detection limit was calculated as three times the standard deviation (3σ) of the ion intensity (n=10) for the mass of each element of the background, in terms of concentration. The detection limitations [ng/g (ppb)] were the following: Ti 0.043, Ge 0.014, Zr 0.013, Nb 0.025, Mo 1.06, Sn 0.030, Hf 0.010, Ta 0.019, and W 0.12.
Considering that the amount of eluent used was 10 cm3 of 3 kmol/m3 nitric acid, based on 3.2, the measured solution can be enriched. Therefore, the lower limit of quantification can be improved.
The results of analyzing high-purity iron SUS-B-97 produced at Der Dillinger Hüttenwerke AG are shown in Table 2. Moreover, Zr of steel JSS 173-7 only for The Japan Iron and Steel Federation steel attestation standard material trace element analysis was measured. Results are shown in Table 3. Both yielded excellent results compared with the indicated value.
| Analyte | Certified value (ppm) | Found (ppm) | |
|---|---|---|---|
| 1 | 2 | ||
| Ti | 0.6 | 0.42 | 0.49 |
| Zr | <0.1 | 0.029 | 0.035 |
| Ta | 0.1 | 0.054 | 0.063 |
| Nb | <0.1 | 0.015 | 0.023 |
With the intention of establishing microelement quantification in steel using solid-phase extraction/ICP–MS with anion-exchange type solid-phase disk as the solid-phase extraction agent, which can be performed simply and rapidly, this study investigated optimum separation conditions and other matters. In this study, the author pursued development of an analytical method using no EDTA or other masking agent like those used in a previous report.21) Results revealed that the target element can be held in the solid-phase disk if the sample solution pH at separation is maintained as higher than pH 1.8. The pH adjustment in this case was conducted by diluting the sample solution with more than about 400 cm3 of water. The target element held in the solid-phase disk was eluted with 10 cm3 of 3 kmol/m3 nitric acid. The method described herein can be used extremely quickly and simply, while involves no cumbersome manipulation. Furthermore, using this method, acids used for decomposition are reduced to the greatest extent possible; particularly, masking agents are not used at all. This method is therefore a “quick”, “skill-free”, and “zero-emission type” analytical method that has been sought in recent years.