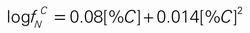Abstract
The nitrogen solubility in liquid Fe–C alloys containing carbon up to 5.2 mass% has been measured under reduced nitrogen partial pressures in the temperature range of 1773–1873 K. Previous studies on the C–N interaction in liquid iron have shown marked disagreement on its temperature dependency and the order of interaction. By the gas-liquid metal equilibration technique using a high frequency induction furnace with an accurate temperature measurement, precise nitrogen solubility data were obtained. The interaction between carbon and nitrogen in liquid iron has been expressed in terms of the first- and second-order interaction parameters. No temperature dependence of these values was observed in the temperature range from 1773 to 1873 K.
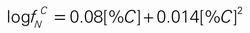
(1.14 ≤ mass% C ≤ 4.95 at 1773 K, 0 ≤ mass% C ≤ 5.2 at 1823–1873 K)