2014 Volume 54 Issue 1 Pages 87-93
2014 Volume 54 Issue 1 Pages 87-93
In order to understand effect of operating conditions on mass transfer between different phases in a mechanically stirred vessel, cold model study was carried out with liquid paraffin as a dispersion phase and ion-exchanged water as a continuous phase. Inner diameter of vessel, D, was varied in conjunction with both depth, H0, as D=H0=400 and 300 mm. Rotation speed, N, was changed between 50–240 rpm, volume ratio, Voil/Vw, of dispersed to continuous phase was 5.9×10–2 and 1.2×10–1.
Liquid/liquid mass transfer rate showed characteristic trend depending on liquid/liquid mixing pattern. It was kept nearly constant at lower level in the region I, monotonically increased in the region II except near the region III and its increasing rate decreased in the region II near the region III. Liquid/liquid mixing pattern was grouped into three regimes. I: the region where liquid/liquid interface did not arrive at the impeller, II: the region where liquid/liquid interface attained at impeller position, III: the region where gas/liquid interface touched impeller.
Under the same supply rate of mixing energy, liquid/liquid mass transfer rate of mechanical stirring corresponded to that of gas stirring at a point in the region II. In the region I and the first half of II, liquid/liquid mass transfer rate of gas stirring is larger than that of mechanical stirring, whereas that of gas stirring is smaller than that of mechanical stirring in the region III and the latter half of II.
Gas/liquid mass transfer rate increased remarkably with an increase in N in the region III.
Slag/metal reaction caused by solid/liquid or liquid/liquid system is one of the most basic and important practices to remove the impurities in steel melt such as gas injection1) or mechanical stirring.2,3,4) In order to research the slag/metal reaction operation, cold model experiment is effective for allowing setup of the wide range of experimental conditions and understanding of the influence of the operation factors easily. Thus, many studies on mechanical stirring practice have been conducted. Kuroyanagi et al.5) and Ito et al.6) showed effect of baffles on solid/liquid stirring, and Sukawa et al.7) and Nomura et al.8) clarified a behavior of particle dispersion in water. Observing solid/liquid flow patterns by cold model experiment, Nakai et al.9) divided the behavior of particle dispersion into three stages, (1): the stage where a vortex does not arrive at impeller position and has no dispersion in liquid, (2): the stage where a vortex bottom exists between top and bottom of the impeller and particles begin to disperse into liquid, and (3): the stage where a vortex bottom attains at the position deeper than the impeller and there has complete dispersion in liquid. They confirmed that the stage (3) is desirable to accelerate solid/liquid reaction by hot model experiment. However, there are few studies on the relation between liquid/liquid mass transfer rate and its mixing pattern.
In this study, to find out the effects of operating factors and mixing pattern10) on liquid/liquid and gas/liquid mass transfer rate, cold model experiments were carried out with liquid paraffin as dispersed phase and ion-exchanged water as continuous phase in a mechanical stirred vessel. Additionally, liquid/liquid mass transfer of mechanical stirring was compared with that of gas stirring under the equal supply rate of mixing energy.
Schematic view of an experimental apparatus is shown in Fig. 1. Liquid paraffin as dispersion phase and ion-exchanged water as continuous phase were charged at total bath depth, H0 mm in a vessel, inner diameter, D mm and vessel height, L mm.

Schematic view of experimental apparatus of mechanical stirring.
Four blades of impeller whose diameter was expressed as di mm, thickness as bi mm and width as wi mm was used as shown schematically in Fig. 2. The impeller was set in the central axis of the vessel.
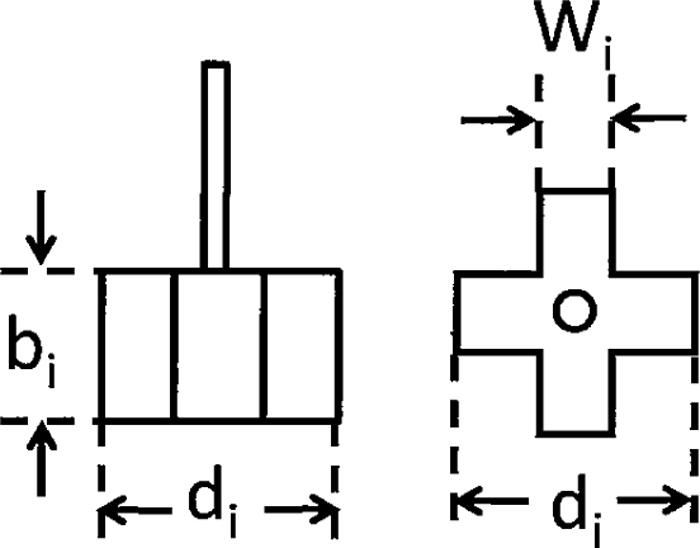
Schematic view of impeller used for experiment.
In order to obtain benzoic acid transfer rate between liquid paraffin and water, temporal change in benzoic acid concentration in water partly transferred from liquid paraffin to water was calculated from the result of electrical conductivity measurement by a conductance meter (Automatic system research, mk-250EC). Liquid temperature was 290±3 K.
On the other hand, a dissolved oxygen analyzer (Wissenschaftlich- Technische- Werkstätten, Multi 3410 SET 5) was used to find oxygen transfer rate between air and water in addition to the liquid-liquid mass transfer experiment. After injecting nitrogen into ion-exchanged water to remove oxygen and injecting air into liquid paraffin to saturate oxygen, oxygen concentration in ion-exchanged water was tracked over time during mechanical stirring. Liquid temperature was 298±3 K.
Experimental conditions including underlined baselines are shown in Table 1. D=H0=400 mm was a standard for vessel and impeller size, (di,bi,Wi) was fixed to (116 mm, 67 mm, 31 mm). Rotation speed, N, was varied from 50 to 240 rpm and distance, H, between free surface of liquid paraffin and impeller upper position from 22 to 313 mm. Volume ratio, Voil/Vw, of dispersed to continuous phase was 5.9×10–2 and 1.2×10–1 (baseline). Density and viscosity of liquid paraffin were 828 kg/m3, 8.20×10–3 Pa·s, respectively. Depths of liquid paraffin and water defined as Hoil and Hw, respectively, were shown in Table 2.
| Variables | |
|---|---|
| Vessel inner diameter, D (mm) | 300, 400 |
| Bath depth, H0 (mm) | 300, 400 |
| Impeller diameter, di (mm) | 116 |
| Impeller thickness, bi (mm) | 67 |
| Impeller width, wi (mm) | 31 |
| Rotating speed, N (rpm) | 50–240 |
| Impeller depth, H (mm) | 22–313 |
| Ratio of dispersion phase to water volume, Voil/Vw (–) | 5.9 × 10–2, 1.2 × 10–1 |
| Dispersion phase | liquid paraffin |
| Continuous phase | Ion-exchanged water |
| Voil/Vw (–) | D = H0 (mm) | Hoil (mm) | Hw (mm) |
|---|---|---|---|
| 5.9 × 10–2 | 400 | 22 | 378 |
| 1.2 × 10–1 | 400 | 42 | 358 |
| 1.2 × 10–1 | 300 | 31 | 269 |
In the previous paper,10) we described that three types of liquid/liquid mixing pattern were observed as shown schematically in Fig. 3 and varied depending on operating factors such as N, Hand Voil/Vw. I (the left side of Fig. 3) is the region where each liquid phase exists separately and has no dispersion, II (the middle of Fig. 3) is the region where vortex of dispersed phase (liquid/liquid interface) arrives at impeller position and its dispersion starts into continuous phase, and III (the right side of Fig. 3) is the region where gas/liquid interface in addition to liquid/liquid one arrives at impeller position and dispersion occurs hard. Moreover, it was also found that the mixing pattern transited from I to II, and from II to III according to an increase in N and a decrease in H.

Schematic view of mixing pattern.
According to double film theory, mass transfer rate of benzoic acid from liquid paraffin to water is given by
| (1) |
Mass balance of benzoic acid is expressed by,
| (2) |
| (3) |
| (4) |
| (5) |
Solving Eq. (4) under an initial condition of Cw= 0,
| (6) |
Kwa is obtained from a temporal change in Cw and Eq. (6). h* value was 0.445 from Voil=2.22×10–3 m3, Vw=1.90× 10–2 m3 and α=3.48×10–3 kg.
On the other hand, Kw is given by
| (7) |
The relation between the overall capacity coefficient, Kwa, of benzoic acid transferred from liquid paraffin into water and H for N=146 and 189 rpm are shown in Fig. 4. Voil/Vw was fixed to 1.2×10–1. Kw and a in the symbol of overall capacity coefficient denote overall mass transfer coefficient and liquid/liquid interfacial area, respectively. Mass transfer rate increased with an increase in N. Depending on mixing pattern described in Fig. 3, distinct change in kia was found as follows. Kwa was almost kept constant for different H in the region I where mass transfer rate was less affected by liquid/liquid interface because it moved like rigid body. On the other hand, Kwa began to increase with the decrease in H in the region II and the increasing rate of kia became lower toward the region III and furthermore in the region III. The reason why Kwa increased in the region II can be attributed to the fact that liquid/liquid interfacial area increases with the increase in dispersion of liquid paraffin into water. Moreover, the decrease in the increasing rate of Kwa in the region II near III as well as III is because most liquid paraffin has already dispersed into water and the increase in liquid/liquid interfacial area decreases.
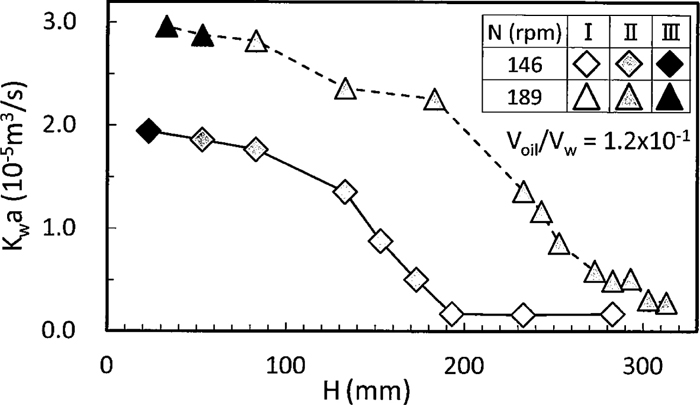
Relation between Kwa and impeller position.
The relation between Kwa and H for Voil/Vw=1.2×10–1 and 5.9×10–2 is shown in Fig. 5 where N was fixed to 146 rpm. Mass transfer rate increased with an increase in Voil/Vw for the equal N and H, which seems to be caused by the fact liquid/liquid interfacial area increases with the increase in Voil/Vw as dispersion phase volume becomes large. The dependence of Kwa on the mixing pattern was similar between Figs. 4 and 5.

Relation between Kwa and impeller position.
The relation between Kwa and N for H=83 and 233 mm is shown in Fig. 6 where Voil/Vw of liquid/liquid was fixed to 1.2×10–1. Mass transfer rate increased with an increase in N because the it caused the transition of mixing pattern to I→II→III.
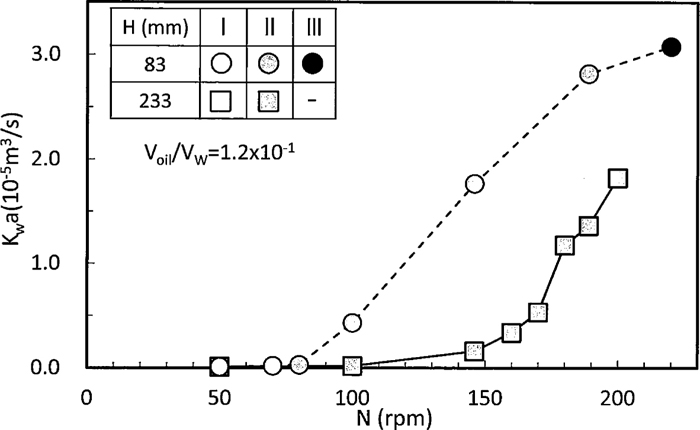
Relation between Kwa and rotating speed.
The calculated value of supply rate, εi, of mixing energy which will be described in section 3.4 became 1.18×10–2 W/kg for N=146 rpm and D=H0=400 mm. On the other hand, N of the same εi value was 117 rpm for D=H0=300 mm. Figure 7 shows that the relation between Kwa/Vw and H for the above two vessels. Voil/Vw was kept to 1.2×10–1, although liquid volume between two vessels was different from one another. Kwa values of two vessels were almost equal in the regions II near III and III, and differed in the region I and II except near III. Assuming that liquid paraffin is fully dispersed into water at the same particle radius, r and number of particles, n, a and Vw become to 4πr2n and (4πr3n/3)/(1.2×10–1), respectively. Therefore, the following equation is given,
| (8) |
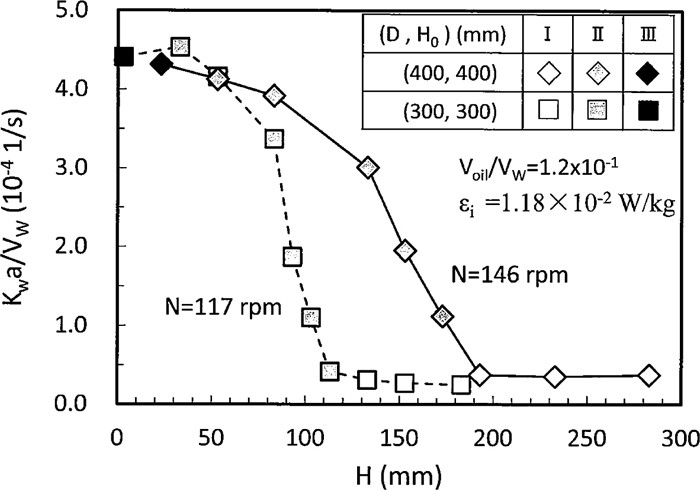
Relation between Kwa/Vw and rotating speed.
A multi-regression analysis was applied to the relation between Kwa and operating variables for the region II. The equation became as follows:
| (9) |
Supply rate of mixing energy of gas stirring expressed as εg (W/kg) is given by Eq. (10),
| (10) |
On the other hand, supply rate of mixing energy of mechanical stirring expressed as εi (W/kg) is given by Eq. (4),12)
| (11) |
| (12) |
The variables of A, B, P, b’ and Re in Eq. (12) are calculated from the following Eqs. (13), (14), (15) ,(16) ,(17).
| (13) |
| (14) |
| (15) |
| (16) |
| (17) |
Both of εg for Q=10 and Q=20 L/min were calculated from Eq. (10) for H0=0.4 m, H=0.28 m, Voil/Vw=1.2×10–1 and T=298 K. Rotation speed for mechanical stirring was obtained by substituting εi values corresponding to the calculated εg ones into Eqs. (11), (12), (13), (14), (15), (16), (17). As shown in Table 3, the rotation speeds, N=146 and 189 rpm correspond to gas flow rates, Q=10 and 20 L/min at H=0.28 m, respectively.
| Q (L/min) | εg (W/kg) | N (rpm) | εi (W/kg) |
|---|---|---|---|
| 10 | 1.18 × 10–2 | 146 | 1.18 × 10–2 |
| 20 | 2.36 × 10–2 | 189 | 2.35 × 10–2 |
Comparison between Kwa of mechanical stirring and gas stirring for the same supply rate of mixing energy is shown in Fig. 8. Gas stirring was carried out through two-holes nozzle immersed into water and air was blown horizontally. Kwa values of mechanical stirring in the region I and up to a point of the region II was smaller than those of gas stirring, whereas they were larger than above the point of the region II and the region III. From the standpoint of slag/metal reaction practice carried out under high temperature, the region III may have potential to damage an impeller due to its partial exposure to atmosphere. Therefore, the mechanical stirring in the region II closed to the region III is more preferable in order to support enhancement in slag/metal mass transfer rate and stable slag/metal operation at the same time.

Comparison of Kwa between mechanical stirring and gas injection for the equal energy supplied rate into bath.
In a mechanical stirring practice, oxygen transfers from air to water by means of two routes: one is that oxygen in air moves into water directly and the other that oxygen moves through liquid paraffin. Thus, oxygen balance in water is expressed by Eq. (18),
| (18) |
Oxygen balance in liquid paraffin is given by oxygen input from air/liquid interface and oxygen output to water shown in Eq. (19).
| (19) |
| (20) |
Overall gas-liquid mass transfer coefficient, KG-L (m/s) is given by Eq. (21).
| (21) |
| (22) |
For liquid-liquid mass transfer rate, Kw,L-L is given by Eq. (23),
| (23) |
Thus, Eq. (20) can rewritten for C’*w,L-L, using constant parameters under a given operating factor such as liquid film coefficient of oxygen transfer through liquid paraffin, kL,G-L (m/s), aoil,G-L, Kw,L-LaL-L, C’*oil,G-L (=11.6×10–3 kg/m3), h**(=1.80), and time-dependent C’w.
| (24) |
When resistance to oxygen transfer between gas and liquid paraffin is ignored compared with that between liquid paraffin and water, that is, koil,G-Laoil,G-L»Kw,L-Laoil,L-L, C’*w,L-L, Eq. (24) can be expressed as Eq. (25).
| (25) |
According to the visual observation, water contacted with air directly in the region III as well as with liquid paraffin, whereas it did not meet air in the regions I and II. Thus, the first term of the right side in Eq. (18) can be ignored for the region I and II as follows:
| (18’) |
By substituting Eq. (24) into Eq. (18’), it can be integrated with C’w=C’0,w at t=0.
| (26) |
| (27) |
When koil,G-Laoil,G-L/h** approaches infinite, resistance of oxygen mass transfer between gas and liquid does not exist and α becomes unit.
For the region III, Eq. (18) can be integrated with C’w=C’0,w at t=0.
| (28) |
| (29) |
| (30) |
Typical examples of temporal change in oxygen concentration in water are shown in Fig. 9. Mixing patterns of N=200 and 300 rpm represented those of the region II and III, respectively. A in Eq. (28) for the region III was obtained from Kw,G-LaG-L calculated by trial-and-error method and αKw,L-LaL-L calculated by extrapolation of the region II. The slopes, (Kw,G-LaG-L+αKw,L-LaL-L)/Vw, of temporal change in oxygen concentration were linear, which means the overall volumetric coefficient was kept constant. Oxygen absorption rate in the region III was faster than that in the region I and II due to gas/liquid mass transfer in addition to liquid/liquid one.
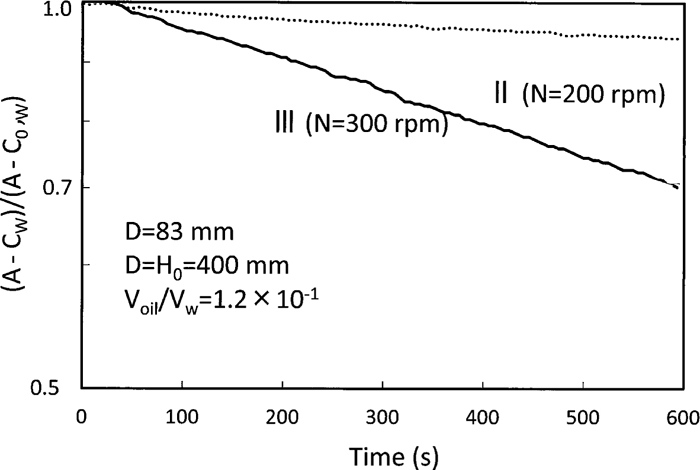
Typical examples of temporal change in oxygen concentration in water.
The relation between Kw,G-LaG-L+αKw,L-LaL-L and N for H=83 mm and Voil/Vw=1.2×10–1 is shown in Fig. 10 where the other operating factors were a standard, and the vertical axis of the region I and II indicates αKw,L-LaL-L. αKw,L-LaL-L was kept nearly constant from the region I to the first half of the region II and it began to increase from the second half of the region II. αKw,L-LaL-L values in the region III were given by extrapolating αKw,L-LaL-L in the second half of the region as indicated in a dotted line in Fig. 10. It was found that Kw,G-LaG-L+αKw,L-LaL-L of the region III increased in a discontinuous manner.
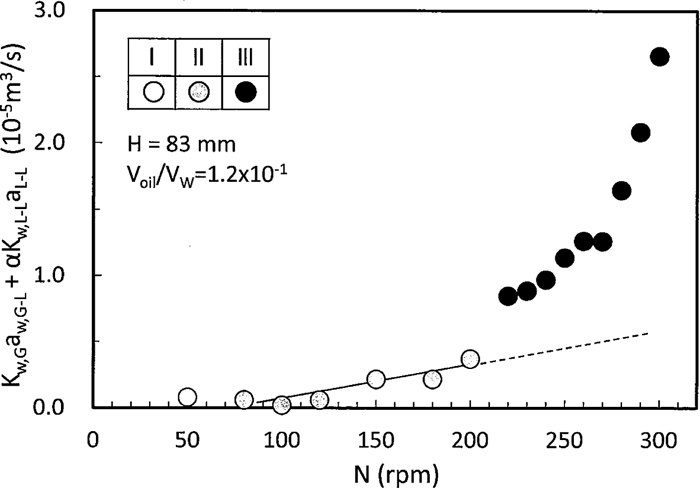
Relation between oxygen absorption rate and rotating speed.
By subtracting the extrapolated αKw,L-LaL-L from the Kw,G-LaG-L+αKw,L-LaL-L in Fig. 10, increasing rate of Kw,G-LaG-L for rotation speed is shown in Fig. 11. As presented in the visual observation, the direct oxygen transfer between immersed air bubbles and water remarkably increased Kw,G-LaG-L in the region III.
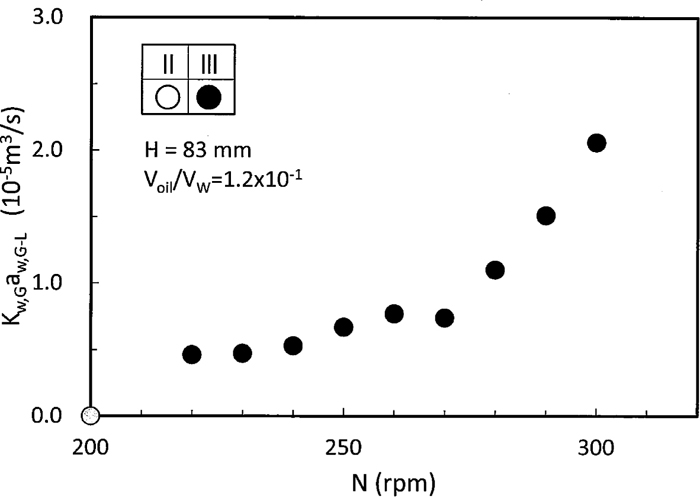
Increase in direct oxygen absorption rate between air and water in the region III.
αKw,L-LaL-L became 0.7×10–5 m3/s at N=200 rpm as shown in Fig. 10, whereas Kwa was estimated to be 2.9×10–5 m3/s under the same experimental condition as known from Fig. 6. The value of Kwa was 4.1 times larger than that of αKw,L-LaL-L. Using Eqs. (7) and (23), kw/(koil/h*) = 0.81 and kw,L-L/(koil,L-L/h**) = 3.2, and assuming that mass transfer coefficient is proportional to square root of diffusivity, Eq. (31) was obtained as follows.
| (31) |
| (32) |
Equation (32) gives koil,G-Laoil,G-L/Kw,L-LaL-L=1.0, which means oxygen transfer rate between air and liquid paraffin is almost the same value as that between water and liquid paraffin at N=200 rpm in the region II.
Cold model study on liquid/liquid mass transfer was carried out with liquid paraffin as a dispersed phase and with ion-exchanged water as a continuous phase in a mechanical stirred vessel.
(1) Liquid/liquid mass transfer rate had almost the same trend depending on mixing pattern. The mass transfer rate was kept nearly constant on a low level in the region I, monotonically increased in the region II except near the region III and the increasing rate was decreased in the region II near the region III and the region III. Here, I is the region where liquid/liquid interface does not arrive on an impeller, II the region where liquid/liquid interface attains at an impeller and a part of liquid paraffin disperses in water and III the region where gas/liquid interface as well as liquid/liquid touches with an impeller and much liquid paraffin disperses into water phase.
(2) Liquid/liquid mass transfer rate increased with a decrease in depth from free surface to impeller position and increases in rotation speed and ratio of dispersion phase volume to continuous one, because these factors accelerated the transitions of mixing pattern from I to II.
(3) A multi-regression equation on liquid/liquid mass transfer rate in the region II was as follows:
(4) Mass transfer rate of gas stirring was faster than mechanical stirring in the region I and the first half of the region II, whereas that of gas stirring was slower than mechanical stirring in the second half of the region II and the region III.
(5) Gas/liquid mass transfer rate increased remarkably with an increase in rotation speed in the region III.
This work was carried out under the project of NEDO (New Energy and Industrial Technology Development Organization), entitled “Research and development project on enhancement of usage of hard-to-use ferrous scrap”.