2014 Volume 54 Issue 10 Pages 2162-2168
2014 Volume 54 Issue 10 Pages 2162-2168
Pyrite cinder contains considerable amount of nonferrous metals and the iron grade of pyrite cinder is low for blast furnace burden, so processing the pyrite cinder has become a significant issue for the utilization of valuable resources and environmental protection. In this study, a technique with high temperature chloridizing-reduction roasting is presented in aim to remove nonferrous metals from iron and produce blast furnace burden with high quality. The effects of chloridizing temperature and time, chlorinating agent dosage, reduction temperature and time are investigated. The optimum process parameters are determined as follows: chloridizing at 1125°C for 10 min, CaCl2 dosage of 2% and reduction at 1100°C and the coal/cinder ratio of 2.5 for 40 min. Under these conditions, the compressive strength of prereduced pellets is 1147N per pellet, the metallization degree is 84.53% and the removal rates of Cu, Pb and Zn are 37.87%, 99.31% and 80.20%, respectively.
As a kind of significant secondary resource, pyrite cinder contains not only abundant iron but also considerable copper, lead, zinc, etc. Tens of millions of tons of pyrite cinder is discharged from chemical industry annually in China,1) and more than one hundred million tons of accumulated waste slag is in storage, which results in occupation of land and pollution.1,2) As the product of high temperature treatment, pyrite cinder has the distinctly different properties from the natural ore. In pyrite cinder, iron has closely associated with copper, lead and zinc, which makes it difficult to utilize. Only a small amount is used as additives in cement, paving, brickmaking and auxiliary additives.3,4,5,6)
To date, an increasing amount of pyrite cinder containing low nonferrous metals and high grade of iron is used as blast furnace burden by sintering and pelletizing.7,8,9) However, sintering and pelletizing requires high iron grade, especially oxidized pellets (the grade of iron should be more than 63%). Also, the nonferrous metal content in pyrite cinder must be very low because it is hard to remove the nonferrous metals by sintering and pelletizing. According to the literature,10,11,12,13) high temperature chloridizing roasting technology has been successfully applied to recover iron and valuable nonferrous metals but mainly focused on the processing high iron grade pyrite cinder.
It is necessary to explore a feasible process to recover iron and nonferrous metals from pyrite cinder with low iron grade and high content of nonferrous metals. Reduction roasting in the rotary kiln has been identified as a significant method to recycle lead and zinc from metallurgical solid wastes and improve the iron grade of blast furnace burden.14) Therefore, preparation of prereduced pellets by pyrite cinder will be investigated by simulating grate–kiln process, including high temperature chloridizing roasting and reduction at a laboratory scale.
The chemical composition and the size distribution of the pyrite cinder mixture are listed in Tables 1 and 2 respectively. Pellet feed contain 61.24% Fe, which is too low for the preparation of oxide pellets and high content of nonferrous metals is not helpful for ironmaking. Thus, it is significant to remove the copper, zinc and lead and upgrade iron. In addition, 6.43% of SiO2 in the pellet feed is unfavorable to ironmaking, but SiO2 favors chlorination by promoting the formation of HCl and Cl2.13) The pyrite cinder mixture is coarse for pelletizing with the size of 77.75% passing 0.075 mm and 60.20% passing 0.045 mm. In order to improve the pellet quality, it is essential to pretreat pellet feed before balling. The morphology of pyrite cinder mixture’s particles under SEM is depicted in Fig. 1, which indicates a porous structure of pyrite cinder particles.
| Fetotal | FeO | Fe2O3 | SiO2 | Al2O3 | CaO | MgO | P | S | Cu | Pb | Zn |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 61.24 | 19.73 | 65.60 | 6.43 | 1.21 | 0.51 | 0.41 | 0.050 | 0.27 | 0.218 | 1.788 | 0.199 |
| +0.150 mm | 0.150–0.106 mm | 0.106–0.075 mm | 0.075–0.045 mm | –0.045 mm |
|---|---|---|---|---|
| 0.51 | 6.02 | 15.72 | 17.55 | 60.20 |

Morphology of pyrite cinder mixture’s particles under SEM (left-SEM×400; right-SEM×1000).
The chemical phase analyses of copper, lead and zinc are shown in Tables 3, 4, 5. Copper mainly exists in the form of copper oxide and lead mainly exists in the form of lead oxide and lead silicate. The existence of zinc is mainly shown as zinc oxide and zinc ferrite, respectively.
| Copper sulphate | Copper oxide | Copper sulphide | Other copper |
|---|---|---|---|
| 3.18 | 50.00 | 6.82 | 40.00 |
| Lead oxide | Lead sulphide | Metallic lead | Lead silicate |
|---|---|---|---|
| 37.43 | 5.31 | 15.64 | 41.62 |
| Zinc sulphate | Zinc oxide | Zinc silicate | Zinc sulphide | Zinc ferrite |
|---|---|---|---|---|
| 3.16 | 11.58 | 3.68 | 7.89 | 73.68 |
The industrial analysis and the size distribution of the bituminous coal are given in Tables 6 and 7 respectively. The bituminous coal which contains 49.74% of fixed carbon, 11.14% of ash and 32.85% of volatile matter is suitable to reduce pellets.15) The total sulfur (0.42%) in coal is slightly high.
| Fcad/mass% | Vdaf/mass% | Ad/mass% | Mad/mass% | Coking index | St/mass% |
|---|---|---|---|---|---|
| 49.74 | 32.85 | 11.14 | 6.27 | 2 | 0.42 |
*St--total sulfur in coal.
| 5–3 mm | 3–1 mm | 1–0.5 mm | 1–0.5 mm |
|---|---|---|---|
| 17.96 | 29.57 | 16.11 | 36.36 |
The tests covered proportioning of raw materials, blending and homogenising, balling in a disc pelletizer, preheating and chloridizing in a tube furnace, and reduction by the bituminous coal to prereduced pellets.
Green balls were made in a disc pelletizer (diameter 1000 mm, rim height 200 mm, angle of inclination 45° and rotating 28 rpm). The finished green pellets were dried in the oven at 105°C for 4 h for further experiments.
Part removal of non-ferrous metals and hardening of dry balls were achieved by preheating dry balls containing chlorides in a tube furnace (an open system in air; Φ 50 × 600 mm; Fig. 2) to simulate strand grate. When the experimental temperature of the tube furnace centre reached to the target value, ten dry balls loaded in a corundum crucible were pushed into the corundum tube. Five minutes were required for moving the corundum crucible into the centre of tube furnace in order to prevent too fast heating rate and obtain high compressive strength of preheated pellets. After staying in the centre for the setting time, the corundum crucible loaded with pellets was drawn out during 5 min and cooled in air. The removal rate of nonferrous metals was worked out by Eq. (1) based on the chemistry of preheated pellets and dry pellets.
| (1) |

Schematic diagram of furnaces (left-tube furnace, right-shaft furnace).
The reduction tests were performed in a shaft furnace (Fig. 2) to simulate rotary kiln. The upper end of corundum tube was covered with a refractory block during reduction in order to maintain enough CO concentration in the shaft furnace (Φ 80 × 800 mm). The stainless steel jar loaded with 15 preheated pellets was put into the centre of shaft furnace to be preheated for 5 min and then taken out. After being charged with the bituminous coal, the jar was moved into the shaft furnace immediately again for reduction. The prereduced pellets were cooled by nitrogen gas to prevent deoxidizing when the reduction finished.
The quality of prereduced pellets was valued by using metallization degree. Compressive strength of pellets was measured by using an intelligent compressive strength instrument (type: ZQYC) according to the standard of ISO4700-2007. Chemical analyses and other test data were obtained by corresponding ISO standards. Microstructure of pellets was measured by using optical microscopy. True density of preheated pellets and prereduced pellets were tested by pycnometer method (YB373-75). Apparent density and volume of pellets were tested by water immersion.16) The porosity and the volumetric shrinkage ratio were calculated by Eqs. (2) and (3), respectively.
| (2) |
| (3) |
Figure 3 illustrates the effect of preheating temperature on compressive strength, residual Cl content and removal rates of nonferrous metals of preheated pellets. The compressive strength of preheated pellets dramatically increases with the increasing preheating temperature, because high temperature can promote the formation of Fe2O3 microcrystals which result in high compressive strength. The removal rates of copper and zinc have a slightly rise with the increasing temperature in the range of 1025°C and 1125°C, while the removal rate of lead shows an obvious drop when the temperature is above 1075°C. The highest removal rates of Cu, Pb and Zn are 37.76%, 94.53% and 26.85%, respectively. According to thermodynamic calculation, more HCl and Cl2 are produced (Fig. 4) at higher temperature. The high temperature also promotes chlorination reactions (Figs. 5 and 6). However, preheating at a high temperature dramatically raises the saturated vapor pressures of CaCl2,17) which accelerates the volatilization of CaCl2 in the open system. Therefore, the amount of HCl and Cl2 for chlorination reactions decreases, leading to the drop in removal rates of copper, lead and zinc. Figure 3(b) indicates that lead is the easiest to remove, following by copper and then zinc, which agrees with the thermodynamic calculation (Figs. 5 and 6). Figures 5 and 6 reveal that acid oxides, such as SiO2, dramatically improve the chlorination reaction in the chloridising roasting, which effectively promote the removal of nonferrous metals. Moreover, the values of Gibbs free energy of iron oxide chlorination reactions are positive, and the values of Gibbs free energy of nonferrous metals chlorination reactions are negative (Figs. 5 and 6), which is the reason of separation of copper, zinc and lead form iron. The residual Cl content of preheated pellets reduces with the increasing temperature (Fig. 3(b)), because high temperature accelerates the volatilization of CaCl2 and improves the chlorination of nonferrous metals.

Effect of preheating temperature on compressive strength, residual Cl content and removal rates of nonferrous metals of preheated pellets (2% CaCl2 and preheating for 20 min).

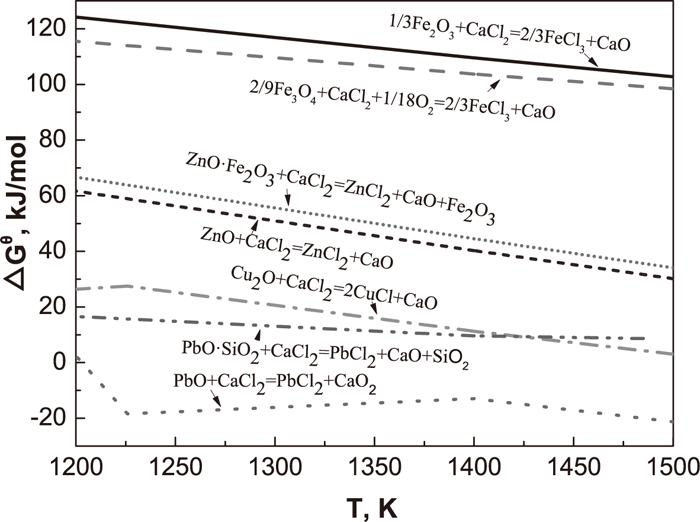
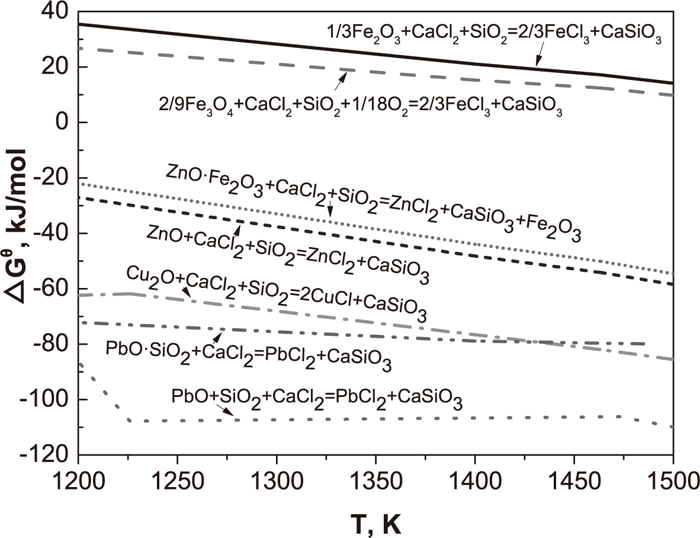
Figure 7 shows the effect of preheating time on compressive strength, residual Cl content and removal rates of nonferrous metals of preheated pellets. The compressive strength of preheated pellets dramatically increases with the increasing preheating time which enhances the oxidation of Fe3O4, solid phase diffusions and the formation of Fe2O3 microcrystals inside pellets.18) As the chlorination time increases, the removal rate of Zn slightly increases and the removal rate of Cu is constant, while the removal rate of lead visibly increases within 10 min (Fig. 7(b)). The residual Cl content of preheated pellets decreases with the increasing time (Fig. 7(b)). When the preheating time is 5 min, the residual Cl content is only 0.035%, which shows the chlorination reactions have almost finished. To sum up, the preheating time should be no less than 10 min.
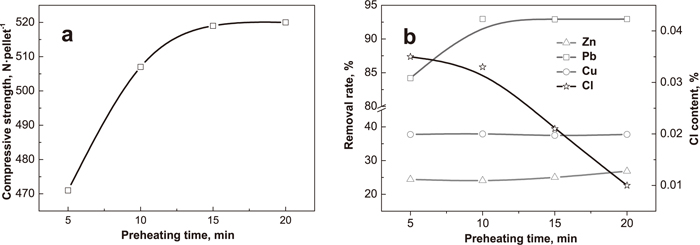
Effect of preheating time on compressive strength, residual Cl content and removal rates of nonferrous metals of preheated pellets (2% CaCl2 and preheating at 1125°C).
The effect of CaCl2 dosage on compressive strength, residual Cl content and removal rates of nonferrous metals of preheated pellets is demonstrated in Fig. 8. The compressive strength shows a significant increase with the increasing CaCl2 dosage, because CaCl2 liquid can improve the solid phase diffusions and the realignment of particles in pellets.18) However, the compressive strength remarkably decreases when the CaCl2 dosage is more than 1%, due to the high content of CaCl2 volatilization leads to the low content of O2 in pellets. The low content of O2 results in low degree of Fe3O4 oxidation in pellets (Fig. 8(a)), which causes the concentric cracks in pellets, so the compressive strength decrease.
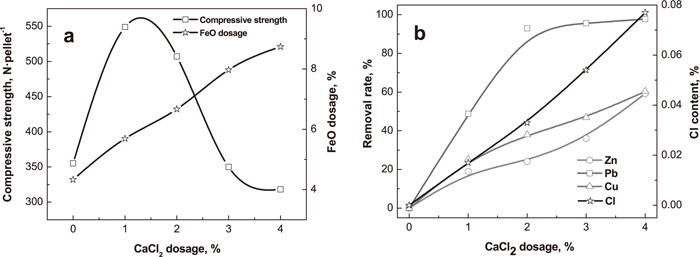
Effect of CaCl2 dosage on compressive strength, residual Cl content and removal rates of nonferrous metals of preheated pellets (preheating at 1125°C for 10 min).
The removal rates of Cu, Pb and Zn dramatically increase with the increasing CaCl2 dosage (Fig. 8(b)), because high CaCl2 dosage can enrich the amount of HCl and Cl2 for chlorination. Therefore, the dosage of Cl has a key effect on the chlorination of Cu, Pb and Zn, especially Cu and Zn. As the CaCl2 dosage increases, the residual Cl content of preheated pellets rises (Fig. 8(b)). When the dosage of CaCl2 is 4%, the content of Cl is only 0.077%.
The results of the optimization of preheating time, preheating temperature and CaCl2 dosage show that volatilization of nonferrous metals and hardening of green balls can be accomplished in the strand grate synchronously, and the CaCl2 dosage should be less than 3% because of the low compressive strength of preheated pellets. The remained zinc and lead must be removed in the next reduction procedure.
3.2. Reduction of Preheated PelletsThe effect of reduction temperature on metallization degree, compressive strength and removal rates of nonferrous metals is depicted in Fig. 9. The compressive strength dramatically increases with the increasing reduction temperature, because the high temperature can improve the development of metallic iron grain and the connection of metallic bonding bridge, which is beneficial to the growth of metallic iron particles (Fig. 10). Especially, the reticular metallic iron crystal is formed when the temperature is above 1075°C, which greatly strengthens the compressive strength. In another hand, both porosity and volume of prereduced pellets decrease with the increasing temperature (Fig. 11), making the prereduced pellets denser, so the compressive strength of prereduced pellets soars.
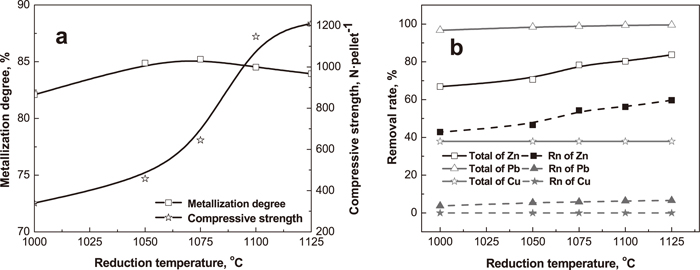
Effect of reduction temperature on metallization degree, strength of prereduced pellets and removal rates of nonferrous metals (2%CaCl2; preheating at 1125°C for 10 min; reducing at coal/cinder ratio of 2.5 for 40 min; solid curve: total removal rates of non-ferrous metals including chlorination and reduction process; dot curve: non-ferrous metals just removed by reduction).

Microstructure of prereduced pellets at different temperatures (metallic iron-bright white, wustite-white; reflection 500 times; a: 1050°C, b: 1075°C, c: 1100°C, d: 1125°C).
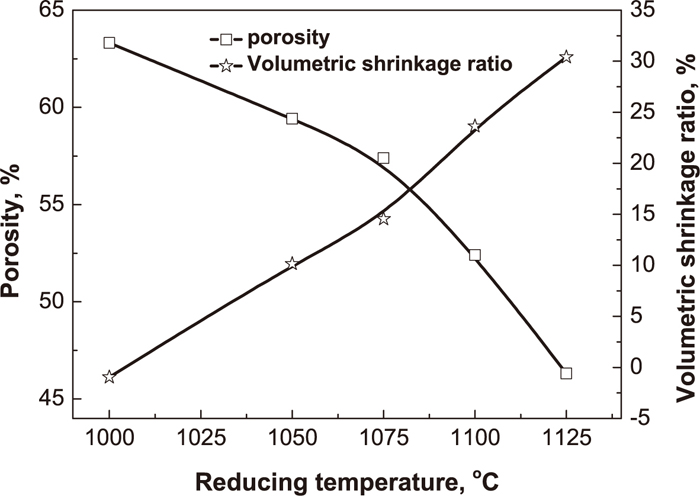
Effect of reduction temperature on porosity and volumetric shrinkage ratio of prereduced pellets.
The metallization degree has a slight rise with the increasing reduction temperature, due to increase of CO concentration which is enhanced by Boudouard reaction. When the reduction temperature exceeds 1075°C, the metallization degree reduces, because reticular metallic iron crystal weakens CO diffusion in prereduced pellets.
The removal rates of Zn and Pb are improved by the increasing reduction temperature (Fig. 9(b)), because the Boudouard reaction responds stronger at higher reduction temperature, which promotes the reduction of zinc and lead compounds. However, the reduction temperature has little influence on the removal of copper, and the total removal rate of copper is only 37.89% (Fig. 9(b)). Under the reduction atmosphere, copper oxide is easy to be reduced to metallic copper which will remain in the prereduced pellets, restricting the removal of copper, further.
The effect of reduction time on metallization degree, compressive strength and removal rates of nonferrous metals is shown in Fig. 12. The metallization degree of prereduced pellets dramatically increases with an increase in reduction time, especially at the initial reduction stage, because the preheated pellets produced by pyrite cinder possess high porosity (Fig. 13). When the reduction time exceeds 40 min, the metallization degree slowly increases.
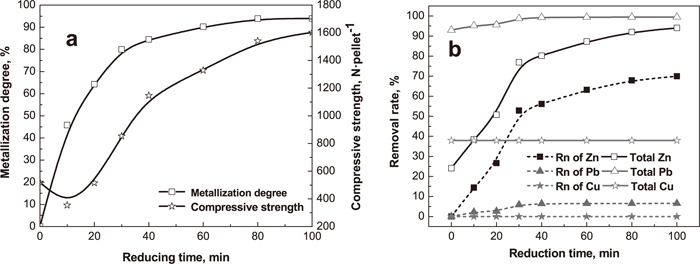
Effect of reduction time on metallization degree, compressive strength and removal rates of nonferrous metals (2%CaCl2; preheating at 1125°C for 10 min; reducing at coal/cinder ratio of 2.5 and 1100°C; solid curve: total removal rates of non-ferrous metals including chlorination and reduction process; dot curve: non-ferrous metals just removed by reduction).
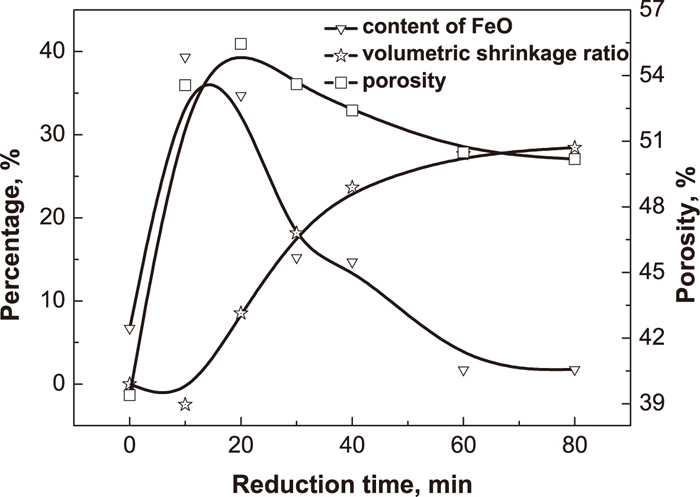
Effect of metallization degree, FeO content and volumetric shrinkage ratio of reduced pellet on compressive strength at different reduction times.
The compressive strength of prereduced pellets drops from 507 N/pellet to 354 N/pellet when reducing for 10 min. Nevertheless, the compressive strength of prereduced pellets soars when the reduction time extends. With reducing for 20 min, the compressive strength of prereduced pellets quickly recovers to 515 N/pellet. At the initial stage of reduction, iron oxide’s crystal form transition leads to drop of pellet’s compressive strength. When the preheated pellets were reduced for 10 min, the FeO content increases to 39.34% and the volume of prereduced pellets expands to 102.48%. With the reduction extending, good reducibility of preheated pellets promotes the formation of metal iron layer outside pellets (Fig. 14), which restrains the reduction swelling and degradation of prereduced pellets. In addition, the porous structure of pyrite cinder (Fig. 1) also alleviates the reduction swelling of prereduced pellets. Thus, the volume of prereduced pellets begins to shrink after reducing for 20 min (Fig. 13). Metallic iron grain begins to develop into reticular metallic iron crystal in middle layer when reducing for 20 min (Fig. 14). With the increasing reduction time, reticular metallic iron crystal continues to develop, and the size of metallic iron particles signally grows up (Fig. 14), which cause the compressive strength of pyrite cinder pellets dramatically increases during reduction. Good reducibility, volume shrinkage, porosity decrease and the reticular metallic iron crystal favour to increase the compressive strength of pyrite cinder prereduced pellets.
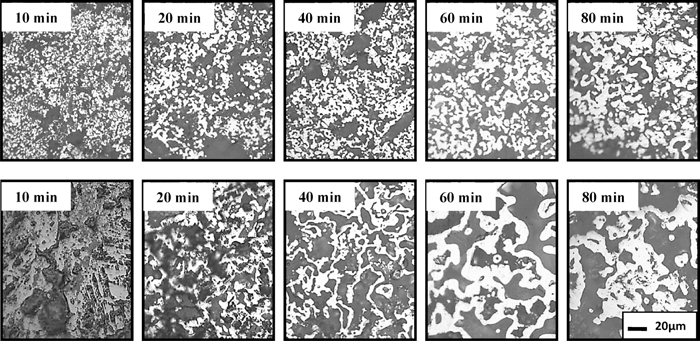
Microstructure of prereduced pellets (above-outer layer, below-middle layer; metallic iron-bright white, wustite-white; reflection 500 times).
The removal rates of Zn and Pb are improved with the long reduction time, especially Zn (Fig. 12(b)). When the reduction time increases from zero to 40 min, the removal rate of Zn increase from 24.08% to 80.20%, and the removal rate of Pb increases form 92.96% to 99.31%. When the reduction time is extended, the removal rate of Pb hardly increases and the removal rate of Zn slightly increases. Whereas, the removal rate of Cu keep steady at 37.89%.
(1) The high temperature chloridizing-reduction roasting technology was developed to utilize pyrite cinder containing nonferrous metals. CaCl2 was added in the pellet feed to partly remove non-ferrous metals, and qualified preheated pellets were obtained for the next reduction process by preheating pellets. In the reduction process, Zn and Pb were further removed and high quality prereduced pellets were acquired.
(2) High temperature chloridizing roasting (preheating roasting) in the grate is one of effective methods to remove nonferrous metals and obtain qualified preheated pellets. Optimized process conditions are as follows: chloridizing at 1125°C for 10 min, and CaCl2 dosage of 2%. Under the conditions, the compressive strength of preheated pellets is 507N per pellet, and the removal rates of Cu, Pb and Zn are 37.89%, 92.96% and 24.08%, respectively.
(3) Reduction roasting in the rotary kiln is an efficient way to further remove Zn and Pb, and raise the iron grade. Optimized process conditions are as follows: reduction at 1100°C for 40 min, and the coal/cinder ratio of 2.5. Under the conditions, the compressive strength of prereduced pellets is 1147N per pellet, the metallization degree is 84.53% and the removal rates of Cu, Pb and Zn are 37.87%, 99.31% and 80.20%, respectively.
The project was sponsored by the Basic Research Program of Jiangsu Province (No. BK20140337 and No. BK20140334).