2014 Volume 54 Issue 10 Pages 2196-2205
2014 Volume 54 Issue 10 Pages 2196-2205
In the smelting cyclone of HIsarna process, both thermal decomposition and gaseous reduction of iron ore contribute to the expected pre-reduction degree about 20%. However, the fine ore reduction and melting process in the smelting cyclone is extremely fast and it is very difficult to differentiate between the thermal decomposition and gaseous reduction. This study focused on the thermal decomposition mechanism of the fine iron ore under different conditions. Firstly, the theoretical evaluation has been conducted based on the thermodynamics, and then the laboratory investigation was conducted in three stages with three reactors: the TGA-DSC, the electrically heated horizontal tube furnace and the High-temperature Drop Tube Furnace (HDTF). According to the experimental results of the first two stages and the theoretical evaluation, it was found that the temperature of intensive thermal decomposition of Fe2O3 in the inert gas environment is in the range of 1473–1573 K, while the thermal decomposition of Fe3O4 could be sped up when the temperature is above 1773 K in the inert gas. Temperature plays an important role in the thermal decomposition degree and reaction rate. Finally, it was found that the thermal decomposition of the individual iron ore particles took place very rapidly in the HDTF and no significant influence of the particle size and residence time (t ≤ 2020 ms) on the equivalent reduction degree could be observed, when the particle diameter was smaller than 250 μm in the CO2 gas.
Nowadays the blast furnace process is still the primary iron making technology. For achieving a more efficient operation from an energy and economic point of view, various revolutionary technologies are now developed as alternative ironmaking processes such as COREX and FINEX already in commercial operation, Circosmelt and HIsarna1,2) under development. HIsarna, as shown in Fig. 1, is one of these promising technologies under intensive development, based on smelting reduction principles. It is the technology which has the potential to reduce emissions of carbon dioxide (CO2) compared to the blast furnace steelmaking route by more than 50%.3) The core of the process consists of three reactors, a smelting cyclone, a smelting reduction vessel (SRV) and a coal pyrolyser. Due to the high temperatures in the furnace, fine iron ore particles are melted in the smelting cyclone. The melt is collected on the water-cooled sidewalls of the cyclone section, runs down the wall and drops into the liquid bath in the SRV where the final reduction is completed. The original iron ore particles are injected into the cyclone reactor and pre-reduced to a reduction degree of about 20% through thermal decomposition and reduction by the post-combustion gases arising from the SRV. Up to now, most of the previous studies4,5,6) focused on the mechanisms of the gas-hematite reaction at low temperature and the gas-wüstite reaction at high temperature with the background of the blast furnace process. The hematite decomposition was usually neglected because of the requirement of the higher temperature than gas-hematite reduction.
The smelting cyclone of the HIsarna process is a complicated high temperature reactor. In the smelting cyclone, the temperature is extremely high and the size of the iron ore particles is very small. The smelting cyclone provides a suitable environment for the thermal decomposition of iron ore particles. Although oxygen is injected into the cyclone together with iron ore particles, it preferably reacts with carbon monoxide and hydrogen rapidly and generates sufficient energy to produce a large amount of energy which heats the particles and the gas quickly. The fine iron oxide particles in the smelting cyclone experience a series of physical and chemical changes including rapid heating up, thermal decomposition, gas-solid particle reduction, melting, and gas-molten particle reduction. For that all the processes are extremely fast and it is very difficult to differentiate between the thermal decomposition and gaseous reduction. It is a fundamental question whether the thermal decomposition of hematite in the cyclone could be an important factor of determining the pre-reduction degree (about 20%) of iron ore. From the thermodynamics point of view, the products of the thermal decomposition and reduction are the same because both of the processes remove oxygen from the high oxygen concentration iron oxides and generate the low oxygen concentration iron oxides. However, from the kinetics point of view, the required reaction conditions and reaction rates are different. In order to obtain a comprehensive and clear understanding of the iron ore behaviour in the smelting cyclone, the laboratory experiments on thermal decomposition of iron ore particles has been carried out. On the other hand, as that the smelting cyclone and SRV have a close contact and the reactions in the smelting cyclone would directly affect the operation parameters in the SRV. Therefore, the kinetic study of the thermal decomposition of iron ore under the conditions of the smelting cyclone will be helpful to optimize the whole HIsarna process.
There are three main forms of iron oxides: hematite (Fe2O3), magnetite (Fe3O4) and wüstite (FeO), and their melting points are 1838 K, 1870 K and 1644 K, respectively. According to the literature, the thermal decomposition of hematite ore has been mentioned in some laboratory experiments. For example, Gilles9) stated that the highest oxide form is unstable above 1810 K and it decomposes into gaseous oxygen and an oxide containing 71.6% iron which is close to the composition of magnetite. This reaction was also mentioned in the study of Nakamura et al.10) in 1981. A laboratory-scale test was made, in which iron oxide contained in a water-cooled crucible was melted and reduced by using 10–15% H2–Ar transferred arc plasma. It was stated that at high temperatures, the oxygen removed by thermal decomposition before the start of the reduction corresponds to a degree of reduction of about 18%. The equation for calculating the equivalent reduction degree R due to thermal decomposition11) and thermal decomposition reactions of iron oxides are as follows.
| (1) |
| (2) |
| (3) |
| (4) |
| (5) |
| (6) |
ΔGo – the standard Gibbs free energy (J/(mol·K))
po – the reference pressure in the system (Pa)
po2 – the partial pressure of oxygen (Pa)
Rg – the universal gas constant (Rg =8.314 J/(mol·K))
Since the reaction furnace in this study is an open system, the reference pressure (po) of the system is about 1 atm. Figure 2 was made according to Eq. (5) based on the reference pressure of 1 atm. It shows that the equilibrium partial pressure of oxygen (po2) as a function of temperature for hematite, magnetite and wüstite, increases with the increase of temperature. The calculation results for the case of hematite show that when the po2 in the system is 0 Pa, the reaction would take place at room temperature. It was found that at 1473 K, the thermal decomposition of hematite can take place only if the po2 is less than 82 Pa which is about 0.08% of 1 atm. At 1573 K, the thermal decomposition of hematite can take place if the po2 is less than 1107 Pa which is about 1.1% of 1 atm. Figure 2 reveals that above about 1500 K, the equilibrium po2 increases rapidly with the increase of temperature. It means that if the po2 in the reaction system is controlled close to be less than equilibrium partial pressure, the thermal decomposition of hematite will accelerate at above 1500 K. It can also be calculated that the boiling temperature of the thermal decomposition of hematite in an open system is 1783 K, at which temperature hematite could decompose intensively with po2 of 1 atm. The thermal decomposition of magnetite is much more difficult than that of hematite. Although the thermal decomposition of magnetite can also take place when the po2 is 0 Pa, according to the thermodynamic calculation, the equilibrium po2 only has slight increase between 1673 K and 1773 K. However, it has an obvious increase from 1773 K to 1873 K. The boiling temperature of the thermal decomposition of magnetite in an open system is 2186 K. The decomposition of wüstite is the most difficult process as shown in Fig. 2. Below the temperature of 2200 K, the equilibrium partial pressures are almost always kept at the value of zero. If the temperature is 4770 K, the equilibrium po2 can reach 1 atm, which is much higher than hematite (1783 K) and magnetite (2186 K).

Equilibrium of oxygen partial pressure as a function of temperature for hematite, magnetite and wüstite.
The actual equivalent reduction degree of hematite ore caused by thermal decomposition in the cyclone furnace is difficult to estimate because of the complicated environment. The laboratory experimental study of the thermal decomposition of hematite ore has been conducted in three methods with three different reactors, and 4 influential factors (temperature, gas composition, particle size, and residence time) were investigated.
Method 1: Thermal decomposition of hematite ore with a TGA-DSC analyser with an inert gas flow (small sample size: 80 mg; 293–1753 K; po2 ≈ 0 Pa); Aim: observe the thermal decomposition at elevated temperature.
Method 2: Thermal decomposition of hematite ore at different temperatures with an electrically heated horizontal furnace with inert carrier gas (larger sample size: 10 grams; 1673 K, 1773 K, 1873 K; po2 ≈ 0 Pa); Aim: confirm the results with TGA-DSC analyser and obtain the accurate equivalent reduction degree.
Method 3: Thermal decomposition of hematite ore in different inert gas compositions, with different particle sizes, and residence time in a HDTF (individual particles: total sample size of 3 grams; 1550 K, 1600 K, 1650 K, 1700 K, 1750 K, 1800 K; po2 ≈ 0 Pa); Aim: study the in-flight thermal decomposition behaviour and provide more direct information for the HIsarna cyclone reactor.
3.2. Experimental Set-up and Conditions 3.2.1. MaterialsThe raw material of the dry iron ore used in the experiment was provided by Tata Steel in IJmuiden, the Netherlands as shown in Table 1. The hematite content is as high as 94.9%. The raw material was prepared by sieving to get different size groups of particles and then dried to remove the moisture.
| Composition | Mass% | Composition | Mass% |
|---|---|---|---|
| Al2O3 | 0.845 | Mn | 0.147 |
| CaO | 0.015 | SiO2 | 2.800 |
| Fe2O3 | 94.935 | Rest | 1.228 |
| MgO | 0.030 | — | — |
The first stage of the experiments has been carried out by using a thermogravimetric analyzer TGA-DSC (NETZSCH Thermal Analysis STA 409). The inert gas of N2 (purity: 99.999%) was controlled by a mass flow meter to protect the sample. The sample holder is a cylindrical crucible which has the volume of 85 μl. This study focused on the thermal decomposition behaviour of hematite ore at elevated temperature. The experimental conditions are shown in Table 2.
| Experimental condition | Operating parameters |
|---|---|
| Sample | Hematite ore: Fe2O3 94.9% |
| Holding time (h) | 4 |
| Heating rate (K/min) | 10 |
| Particle size (μm) | Hematite ore: 45–53, 125–250 |
| Temperature (K) | 293–1753 |
The sample size in the TGA-DSC analyser is too small (~80 mg) to do chemical analysis or other tests. The experiments were further carried out in the horizontal tube furnace at pre-selected isothermal temperatures as shown in Fig. 3. A larger sample size could be used to obtain an accurate equivalent reduction degree. The experimental conditions are listed in Table 3. The temperature at the isothermal zone was measured by a type S thermocouple. From this experiment, the maximum equivalent reduction degree of hematite ore at a certain temperature and in a certain holding time (keeping po2 ≈ 0) has been obtained. Only the small particle size of hematite ore was used in the experiment. When the holding time is long enough, the particle size doesn’t play an important role in the final equivalent reduction degree. During the experiment, high purity of N2 gas has been used as protection gas. After the experiment, the sample was grinded into powder for the further analysis with chemical titration.
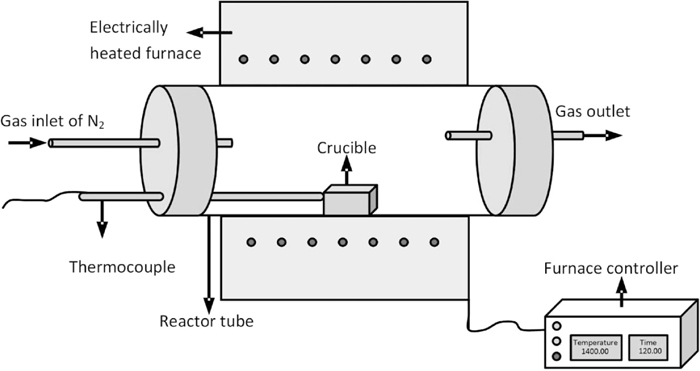
Schematic diagram of the electrically heated horizontal tube furnace.
| Experimental condition | Operating parameters |
|---|---|
| Sample | Hematite ore: Fe2O3 94.9% |
| Holding time (h) | 1, 2, 3 |
| Heating rate (K/min) | 10 |
| Particle size (μm) | Hematite ore: 45–53 |
| Temperature (K) | 1673, 1773, 1873 |
In this study, the thermal decomposition behaviour of the hematite ore has been further explored with the HDTF. The schematic drawing of the experimental set-up is shown in Fig. 4. The laboratory experimental set-up of the HDTF was normally used in the study of coal combustion behaviour.13,14,15) The core of the experimental set-up mainly consists of 6 facilities: an electrically heated tube furnace, a syringe pump particle feeder, a particle injection probe, a sampling probe, a gas pre-heater and a sample collector. The injection probe and sampling probe are made of high temperature resistance stainless steel. The alumina tube with inside diameter of 60 mm, outside diameter of 70 mm and length of 1100 mm was used as the reactor tube (volume: 3.1 L). After the starting of the experiment, a continuous flow of inert gas from the gas cylinder was regulated by a mass flow controller. The typical gas flow rate was 4 L/min. A small part of the gas was separated as carrier gas which was measured by a mass flow meter. The carrier gas flowed into the glass tube of the particle feeder and brought several particles together with it, and then flowed into the water cooled injection probe. The remaining gas was preheated to 773 K, and then introduced into the reactor directly from the top of the furnace. The thermal decomposition took place in the hot zone of the furnace which starts from the tip of the injection probe and ends at the top of the sampling probe. The reacted particles and gas were received by the water cooled and gas quenched sampling probe. Finally, the particles were collected in the sample collector. The temperature distribution at the hot zone is shown in Fig. 5 with three examples: 1600 K, 1650 K and 1700 K.

Schematic diagram of the High-temperature Drop Tube Furnace (HTDF).

Temperature profiles at the hot zone.
The experimental conditions are shown in Table 4. The effects of gas, temperature, residence time, and particle size on the iron ore thermal decomposition have been studied in the HTDF. The inert gas (purity: 99.999%) was changed from the symmetric molecule gas N2 to monoatomic gas Ar, and then to the asymmetric molecule gas CO2. The temperature was changed from 1550 K to 1800 K. The residence time was varied from 210 ms to 2020 ms, which was calculated by applying Newton’s second law of motion and Stokes law to the particles’ motion through fluids. It was assumed that there were three forces acting on a single particle: gravity force, buoyancy force and drag force. The six particle sizes have been tested, which are in the overall range of 38–250 μm. To prevent the thermal shock and extend the service life of the reactor tube and heating elements, the heating rate of the furnace was set to 3 K/min and the cooling rate was set to 2 K/min.
| Experimental condition | Operating parameters |
|---|---|
| Sample | Hematite ore: Fe2O3 94.9% |
| Particle feed rate (g/h) | 1 |
| Gas composition | 100% N2; 100% Ar; 100% CO2 |
| Particle size (μm) | 38–45, 45–53, 53–75, 75–90, 90–125, 125–250 |
| Residence time (ms) | 210, 970, 2020 |
| Temperature (K) | 1550, 1600, 1650, 1700, 1750, 1800 |
Firstly, a series of experiments have been carried out with hematite ore with different particle sizes and heating rates. Figure 6 shows the TG and DSC profiles of one test. The weight of the sample was 80.2 mg and the particle size was in the range of 45–53 μm. The highest temperature achieved was 1753 K and the heating rate was set at 10 K/min. In Fig. 6(a), the temperature history in the furnace is presented by the red line. It was linearly increased to the maximum temperature and then held for 4 hours. There are three steps on the TG curve and two troughs on the DSC curve before the holding time. Due to that the device was not stable at the beginning of the test (0–30 min.), the TG value was a little more than 100%. The total weight loss of the sample is about 4.43%. A slight weight loss appears at the first stage of the heating time (30–120 min.) due to the thermal decomposition of a small quantity of hematite. The thermal decomposition of hematite is endothermic reaction. It is the reason why the DSC curve goes down below zero.

TG-DSC analysis of hematite ore (Heating rate: 10 K/min) (a) as a function of time, (b) as a function of temperature.
The slight weight loss at the beginning of the heating time (30–120 min.) is followed by the sharp weight loss (at about 130 min.). The total weight loss at the end of the stage of sharp weight loss is around 3.55%. Firstly, it indicates that hematite is not stable with the increase of temperature in the inert gas, which is in agreement with the theoretical evaluation perfectly. Hematite started to decompose slowly with the increase of temperature in the first stage (heating time: 0–120 min. and temperature: 298–1473 K), while the decomposition rate was accelerated when temperature was raised above around 1473 K and the sharp weight loss was obtained. Secondly, the stage of sharp weight loss is corresponding to the first big trough on the DSC curve which means that the mass loss was caused by an endothermic reaction of the thermal decomposition of hematite. Further more, the reaction enthalpy of thermal decomposition of 1 mol Fe2O3 is about 78.24 kJ/mol (ΔH298) at 298 K and it increases to 78.71 kJ/mol (ΔH1473) at 1473 K. The reaction heat only has a small increase with the increase of temperature. Therefore, the big trough on the DSC curve at the sharp weight loss stage is caused by the high decomposition rate. Thirdly, it can be calculated that if Fe2O3 decomposes to Fe3O4 completely, the weight loss is about 3.3%. It means that the hematite was probably completely transferred to magnetite at the end of the sharp weight loss. The weight loss of 0.25% (3.55% – 3.3%) is probably caused by the thermal decomposition of magnetite. The second trough shows that the heat absorption of the reaction during this period is less than the heat absorption of the reaction which results in the first trough. The weight loss during the second trough is also not obvious. By the end of the second trough, the weight loss is about 3.74% (3.55% + 0.19%). For that the TGA-DSC analyser is an open system, the small amount of generated O2 could be taken away by inert gas continuously. The oxygen partial pressure in the reaction system can be kept at a very low level. Therefore, the second trough would be caused by the decomposition of magnetite or the evaporation of the other impurities in the iron ore sample. The total weight loss during the rest time is only 0.69% (4.43% – 3.55% – 0.19%), although it has a 4 hours holding time. The result shows that the weight loss decreases slowly after 200 minutes. If all the weight loss is caused by thermal decomposition of iron oxides, the equivalent reduction degree of hematite ore can be roughly estimated to be 14.8%.
From Fig. 6(b), it can be clearly seen that the sharp weight loss stage is exactly marked by the big trough on the DSC curve. According to the analysis of the DSC curve, the onset and end temperatures of the first trough are 1537 K and 1617 K, respectively. The point of 1537 K can be seen as the start temperature of thermal decomposition of the hematite ore at the sharp weight loss stage. In other words, the thermal decomposition rate of hematite is accelerated above 1537 K. The onset and end temperatures of the second trough are 1693 K and 1720 K. The start temperature of intensive thermal decomposition of hematite obtained from experiments is in agreement with the range of the theoretical evaluation. The same conclusion can be obtained that thermal decomposition of magnetite is much more difficult than the hematite.
The larger particle sized hematite ore was also tested with the TGA-DSC analyser as shown in Fig. 7. The particle size is the group of 125–250 μm. The heating rate is 10 K/min. The results confirmed that the thermal decomposition of hematite has a sharp weight loss stage during the heating time. The weight loss of the sharp weight loss stage is about 3.4% and the total weight loss is about 4.6% in the 4 h holding time. In Fig. 7(b), the start temperature of the sharp weight loss stage of hematite is about 1556 K and the start temperature of the second trough on the DSC curve is about 1698 K. The two temperatures are slightly higher than the results in Fig. 6(b). It probably because that the larger particles were heated up more slowly than the small particles in the furnace.

TGA-DSC analysis of hematite ore (Particle size: 125–250 μm) (a) as a function of time, (b) as a function of temperature.
The experiments in the horizontal furnace differ from the TGA-DSC analysis by the amount of the sample and the way of heating (temperature profile). TGA-DSC analysis could give the description of the ongoing process of thermal decomposition, which shows the intensive reaction temperature, exothermic reaction or endothermic reaction, and the weight loss at each moment. However, the more accurate equivalent reduction degree caused by thermal decomposition of hematite ore could not be analysed by chemical analysis, while the experimental study in the horizontal furnace could solve this problem. The larger sample size has been tested in the horizontal furnace. Chemical titration was first carried out with a certified reference material JK 29 consisting of 90.11% magnetite and 7.1% hematite, the components of which have been analyzed by a Swedish institute. Thereafter the samples in this experiment were analyzed with the accuracy of ± 0.2%. More importantly the thermal decomposition experiments in the horizontal tube furnace were conducted at a constant temperature, and the time to reach the reaction temperature is very short (TGA-DSC tests have a constant heating rate of 10 K/min.). The temperature of the sample before pushing to the hot zone of the reactor tube is room temperature. It is similar to the actual situation.
Hematite ore was suddenly positioned at the isothermal zone of the horizontal furnace. According to the thermodynamic theory, for an open system, the released O2 is constantly removed by the flowing inert gas and maintain the real partial pressure of oxygen lower than the equilibrium value in the reactor, so the thermal decomposition reactions would not stop as long as the samples are kept in the isothermal zone. However, according to the results of TGA-DSC analysis, the reaction rate becomes very low after two hours holding time and the sharp weight loss stage was observed during the heating time (before the holding time). In order to obtain the sharp weight loss stage in the horizontal furnace, different holding time of 1 hour, 2 hours and 3 hours at 1673 K were tested to make sure that the total experimental time is long enough. The results shown in Fig. 8 indicate clearly that the sharp weight loss already takes place in the experiment with 1 hour holding time, and the equivalent reduction degree (R) of hematite ore after 2 hours holding time increases slowly. Therefore, the holding time was fixed to 2 hours for the other experiments.

Iron ore equivalent reduction degree in different holding time at T = 1673 K.
The effect of temperature on the equivalent reduction degree is shown in Table 5. In the table, TFe% denotes weight percentage of total iron in the sample, Fe2+%, and Fe3+% are the weight percentages of Fe2+ and Fe3+ in the sample, respectively. According to the composition of the raw material, the elemental Fe is completely in the form of Fe2O3 in the raw material. Therefore the original oxygen percentage in the sample can be calculated with the weight percentage of the total iron in the sample. On the other hand, the oxygen weight loss by decomposition can be calculated with the weight percentage of Fe2+ in the sample. Finally, the equivalent reduction degree can be evaluated by Eq. (1). The rough product composition is calculated by assuming that FeO is generated until the transformation from Fe2O3 to Fe3O4 is completed. According to the assumption and theoretical evaluation, the partially decomposed hematite ore is mainly composed of magnetite and wüstite and the percentage of wüstite goes up with increasing temperature. Generally, the equivalent reduction degree increases with the increase of temperature. The equivalent reduction degree at 1773 K is slightly higher than that at 1673 K by 0.87%. However, it increases strongly from the temperature of 1773 K to 1873 K. At 1873 K, the decomposition reaction of Fe2O3 takes place intensively resulting in 16.4% equivalent reduction degree. It indicates that the thermal decomposition of magnetite becomes faster above 1773 K. It confirms the results of theoretical evaluation as shown in Fig. 2. The hematite ore at 1873 K were melted down completely, while the samples were still in solid state at 1673 K and 1773 K.
| T (K) | t (h) | Chemical titration result (wt%) | R (%) | Composition (wt%)# | Physical state | ||||
|---|---|---|---|---|---|---|---|---|---|
| TFe | Fe2+ | Fe3+ | Fe2O3 | Fe3O4 | FeO | ||||
| 1673 | 2 | 69.1 | 24.3 | 44.9 | 11.7 | 0.0 | 93.0 | 2.3 | Solid |
| 1773 | 2 | 68.9 | 26.0 | 42.9 | 12.6 | 0.0 | 88.9 | 5.8 | Solid |
| 1873 | 2 | 69.5 | 34.3 | 35.2 | 16.4 | 0.0 | 73.1 | 21.4 | Liquid |
With TGA-DSC and horizontal tube furnace, a sharp weight loss stage has been detected during the thermal decomposition of hematite. At the end of the sharp weight loss stage, hematite decomposed to magnetite completely at the experimental temperature about 1723 K of smelting cyclone. It would play an important role in the total pre-reduction degree in the smelting cyclone. However, the residence time of the iron ore particles in the cyclone reactor is much shorter than the time investigated in the TGA-DSC and horizontal tube furnace. Whether the sharp weight loss stage is present in the smelting cyclone and how fast the thermal decomposition will proceed during the sharp weight loss stage were not clear and could not be deduced from the above results. Therefore, the thermal decomposition behaviour of individual particle was further studied with the HDTF, in which the hematite ore particle was injected into the reactor by the injection probe and reaction took place during the flying time in the reactor. The reaction gas in the cyclone reactor mainly consists of CO, CO2, H2, H2O and N2. Through analyzing the off-gas composition from the cyclone reactor, it was found that CO2 is over 60% and N2 is around 5–10%. In this study, both CO2 and N2 were used as inert gas to study the thermal decomposition behaviour of hematite ore. In order to verify the effect of the structure of the gas molecular on the equivalent reduction degree, monoatomic gas of Ar was also used. The equivalent reduction degree of the collected samples was also calculated based on the analysis by chemical titration.
4.3.1. Effect of Temperature and the Type of GasFigure 9 shows the effect of temperature and the type of gas on the thermal decomposition of iron ore particles. The experiments of thermal decomposition of hematite ore in CO2 gas were carried out at 1550 K, 1600 K, 1650 K, 1750 K and 1800 K. The particle size was in the range of 45–53 μm. The particle residence time in the hot zone was maintained at 2020 ms. In order to study the influence of different inert gas on the equivalent reduction degree of iron ore, the experiments have also been carried at three selected temperature: 1650 K, 1750 K and 1800 K in N2 gas and Ar gas.

Thermal decomposition of hematite ore in Ar, CO2 and N2 at different temperatures.
The results show that the equivalent reduction degree of hematite ore increases with the increase of temperature for all inert gases used. But the difference of thermal decomposition between the temperature of 1750 K and 1800 K is quite small. At the three temperatures of 1650 K, 1750 K and 1800 K, the equivalent reduction degrees of hematite ore particles in CO2 gas (solid circle) are much higher than that in N2 gas (solid triangle). The difference of the equivalent reduction degrees in the two gases (CO2 and N2) is about 3–4%. By estimation of the composition of the iron ore sample, it shows that the remaining hematite content in the sample with N2 gas is much higher than that in the sample with CO2 gas. For example, at 1750 K, the Fe2O3 in the product which was decomposed in N2 gas was about 31.2%, while the Fe2O3 in the product which was decomposed in CO2 gas was only about 2.3%. The experimental results in Ar gas (solid square) as shown in the figure are almost the same with the results in N2 gas. It is because CO2 is an asymmetric diatomic molecule, while N2 is a symmetric molecule and Ar is a monatomic molecule. The structure of molecules is important for molecular radiation - emission and absorption which occurs only when an atom makes a transition from one state with a certain amount of energy to a state with lower (higher) energy, respectively. Homonuclear diatomic molecules like N2 and O2 and monatomic molecules like Ar do not have radiative capability for its symmetrical distribution of charges. Compared to N2 and Ar, CO2 has strong thermal radiation capacity. That is also why CO2 gas is the most important greenhouse gas. Therefore a fine particle is heated up more quickly in CO2 gas through thermal radiation than in N2 and Ar gases.
The experiments carried out at 1673 K, 1773 K and 1873 K in the horizontal furnace were selected to compare with the experiments carried out at 1650 K, 1700 K, 1750 K and 1800 K in the HTDF as shown in Fig. 10. It was found that the sharp weight loss stage still exists in the HTDF during the particle flying time through the hot zone. However, the sharp weight loss stage was not completed at 1650 K. The equivalent reduction degree of iron ore in CO2 gas is 7.5% and in N2 gas is 3.5% which are far below the result of 11.7% in the horizontal tube furnace. At 1700 K, 1750 K and 1800 K, the equivalent reduction degrees of 10.8%, 10.9% and 11.0% of iron ore in the HTDF with CO2 are quite close to the result of 12.6% at 1773 K in the horizontal furnace. It indicates that almost the whole sharp weight loss stage observed in TGA-DSC tests was achieved at the temperature from 1700 K to 1800 K in the HTDF, although the residence time of iron ore particles were much shorter than the holding time in the horizontal furnace. This may be caused by two reasons. Firstly, the hematite ore in HTDF is moving individual particles, having much better and more favorite kinetic conditions than in the horizontal tube furnace where they are packed and steady with lower relative moving velocity from the gas. Secondly, the thermal decomposition rate of iron ore at the sharp weight loss stage is extremely fast.

Comparison of thermal decomposition of hematite ore in the horizontal furnace and the HDTF, residence time: 2020 ms in HDTF, 2 hours in horizontal tube furnace.
How fast the thermal decomposition rate of iron ore is at its sharp weight loss stage could not be estimated from the results with TGA-DSC analysis and the horizontal furnace. Although TGA-DSC analysis provided the online report of weight loss as a function of time, the heating rate (10 K/min.) in the TGA-DSC analyzer was different from the heating rate in the smelting cyclone. Therefore, further experiments were carried out in the HTDF with different residence time. The results of chemical analysis of the sample are shown in Table 6. Both N2 gas and CO2 gas were separately used. The residence time of the flying iron ore particle was adjusted by varying the gas flow rate. In the first part, the temperature in the hot zone was set to 1650 K and 1800 K with the inert gas of N2. The particle size was in the range of 45–53 μm. Two residence time of 2020 ms and 970 ms was tested. It was found that the decomposition degree of hematite ore in the N2 gas goes up with the increase of residence time. The equivalent reduction degree in 2020 ms (e.g. 5% at 1650 K) is about 1–1.5% higher than that in 970 ms (e.g. 3.5% at 1650 K) in N2 gas. In the smelting cyclone the bulk gas is mainly composed of CO2 gas which has a strong thermal radiation capacity. Therefore, more experiments have been carried out in CO2 gas. It was found that the equivalent reduction degree did not change by changing the residence time. For example, at 1750 K, the residence time of iron ore particles was verified from 210 ms to 2020 ms, but the equivalent reduction degree of iron ore was all around 10.8% which is very close to 11% (the complete decomposition of Fe2O3).
| Gas | T (K) | t (ms) | Chemical titration result (wt%) | R (%) | Composition (wt%)# | tdu (h) | msample (g) | |||
|---|---|---|---|---|---|---|---|---|---|---|
| TFe | Fe2+ | Fe3+ | Fe2O3 | Fe3O4 | ||||||
| N2 | 1650 | 2020 | 64.4 | 9.7 | 54.7 | 5.0 | 50.5 | 40.1 | ~ 5 | 3–4 |
| 970 | 64.6 | 6.7 | 57.9 | 3.5 | 63.6 | 27.7 | ~ 5 | 3–4 | ||
| 1800 | 2020 | 65.9 | 17.4 | 48.5 | 8.8 | 19.5 | 72.2 | ~ 5 | 3–4 | |
| 970 | 65.5 | 15.5 | 49.9 | 7.9 | 30.8 | 62.1 | ~ 5 | 3–4 | ||
| CO2 | 1750 | 2020 | 66.0 | 21.3 | 44.7 | 10.8 | 3.0 | 88.2 | ~ 5 | 3–4 |
| 1750 | 970 | 67.2 | 21.9 | 45.3 | 10.9 | 2.4 | 90.6 | ~ 5 | 3–4 | |
| 1750 | 210 | 66.8 | 21.2 | 45.6 | 10.6 | 10.0 | 82.5 | ~ 5 | 3–4 | |
*msample is weight of collected samples; tdu means duration of each experiment at the reaction temperature. # calculated based on titration analysis.
Therefore, the residence time in the HTDF has influence on the equivalent reduction degree of iron ore with N2, but has no influence on the equivalent reduction degree of iron ore with CO2. It is mainly caused by the gas radiation properties, which makes the CO2 gas very effective for heat transfer to the particles. In other words, the particle heating rate in N2 gas would be the rate controlling step of the ore thermal decomposition. In the CO2 gas, the studied range of residence time did not affect the equivalent reduction degree, and what’s more the sharp weight loss stage could be partly achieved in 210 ms and no further change of equivalent reduction degree could be detected during the residence time between 210 ms to 2020 ms.
4.3.3. Effect of Particle SizeThe experiments have been conducted with different particle sizes of the hematite ore in CO2 gas. The average temperature of the hot zone was controlled to 1750 K. The experiments were divided into two groups: small particle and large particle. The residence time of the smaller particles was 970 ms and that of the larger particles was 210 ms. The particle sizes in the first (small) group were in the range of 38–45 μm and 75–90 μm and in the second (large) group were in the range of 90–125 μm and 125–250 μm. The results are listed in Table 7. It can be seen that in all the studied particle size groups of 38–250 μm, the equivalent reduction degree of iron ore is in the range of 10.3–11.3%. The small deviation can be neglected. Therefore, the particle size below 250 μm does not have significant influence on the equivalent reduction degree of the hematite ore in the HDTF when the inert gas is CO2. On the other hand, for the difference of particle gravity, the residence time of the larger particles was shorter than the smaller particles. The results proved again that with CO2 gas, residence time is not a significant factor for the thermal decomposition behaviour of fine iron ore particles in the HTDF.
| Gas | dp (μm) | t (ms) | Chemical titration result (wt%) | R (%) | Composition (wt%)# | tdu (h) | msample (g) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| TFe | Fe2+ | Fe3+ | Fe2O3 | Fe3O4 | FeO | ||||||
| CO2 | 38–45 | 970 | 66.6 | 21.8 | 44.8 | 10.9 | 1.7 | 90.4 | 0.0 | ~ 5 | 3–4 |
| 75–90 | 66.0 | 21.0 | 45.0 | 10.6 | 4.7 | 87.0 | 0.0 | ~ 5 | 3–4 | ||
| 90–125 | 210 | 66.5 | 21.4 | 45.1 | 10.7 | 3.5 | 88.5 | 0.0 | ~ 7 | 5–6 | |
| 125–250 | 64.9 | 20.1 | 44.8 | 10.3 | 6.7 | 83.1 | 0.0 | ~ 5 | 3–4 | ||
*msample is weight of collected samples; tdu means duration of each experiment at the reaction temperature. # calculated based on titration analysis.
The sample of 90–125 μm in Table 7 was analysed by XRD to identify the phases in the product as shown in Fig. 11. The figure gives the peak positions and intensities of the identified phases. This confirms fully the analytical results of chemical titration. The main phase in the reacted iron ore sample is magnetite and the phases of hematite and wüstite are either not present (wüstite) or too small to be detected (hematite).

XRD pattern of reacted iron ore sample at 1750 K in CO2.
In this paper, the thermal decomposition behaviour of hematite ore has been studied through theoretical and experimental methods. The experimental study has been carried out with the TGA-DAC analyzer, the horizontal tube furnace and the drop tube furnace (HTDF). The equivalent reduction degree of collected samples from horizontal furnace and HTDF were determined by chemical titration. The results were helpful for the further study of the kinetics of the melting and reduction mechanism of iron ore in the next stage. The conclusions are as follows:
• From the theoretical calculation, Fe2O3 is the most unstable iron oxide among the three iron oxides, while FeO is the most stable one. In an open system with flowing inert gas (the released O2 is removed continuously), the intensive thermal decomposition of Fe2O3 is likely to take place above 1473 K and the thermal decomposition of Fe3O4 is possible to speed up above 1773 K.
• From TGA-DSC analysis, it is found that a sharp weight loss stage appeared on the TG curve in the range of temperature of 1473–1573 K. A big trough was found in the DSC curve at the same time. It was caused by the intensive decomposition of hematite. The result is in accordance with the theoretical estimation.
• From the experimental results in the horizontal furnace, the accurate equivalent reduction degree of hematite at a given temperature and holding time was obtained. The sharp weight loss stage observed in TGA-DSC test was achieved in all the studied temperatures in the horizontal tube furnace in 2 hours. Generally, the equivalent reduction degree of hematite increases with the increase of temperature. No significant difference was observed between the results at 1673 K and 1773 K. However, the equivalent reduction degree of hematite at 1873 K was much higher than that at 1773 K. It confirms that the thermal decomposition of magnetite could be accelerated above 1773 K.
• From the study of in-flight thermal decomposition within HTDF, it was found that sharp weight loss stage which was observed in the TGA-DSC experiments could be partly achieved in the HTDF at high temperatures especially in the CO2 gas. The fine iron ore particles could be heated up faster in CO2 gas than in N2 and Ar gas due to the strong radiation properties (emission and absorption) of CO2 gas.
• No significant influence of particle size and residence time on the equivalent reduction degree could be observed in the HTDF, when the particle diameter is smaller than 250 μm in the CO2 gas. At 1750 K in CO2 gas, the equivalent reduction degree of iron ore in HTDF is around 10.8% which is slightly lower than the value 12.6% obtained in horizontal furnace (holding time 2 hours).
This research was carried out at Delft University of Technology and was financially supported by the Materials innovation institute M2i (www.m2i.nl) under the project M41.5.09327. The authors would like to express their thanks to Mr. Jan van der Stel and Mr. Jeroen Link from Tata Steel Europe (IJmuiden) for fruitful discussions and providing process data for this study. The first author acknowledges the China Scholarship Council (CSC) for providing the scholarship during this research at Delft University of technology.