2014 Volume 54 Issue 11 Pages 2454-2460
2014 Volume 54 Issue 11 Pages 2454-2460
The price of caking coal, which is used in the production of metallurgical coke, has risen in recent years. Also of concern is the amount of CO2 emitted from steel industries, comprising approximately 15% of total CO2 emissions in Japan. Therefore, CO2 emissions from the ironmaking process should be reduced to avoid global warming. In this work, fundamental research is conducted on the effect of adding woody biomass to the properties of coke, with the aim of possibly using woody biomass, which is carbon neutral, as a raw material in coke-making. Experimental results showed that the connectivity between coal particles in the coke sample during carbonization and coke strength drastically decrease by adding woody biomass to caking coal. However, the coke properties of the coke sample with added woody biomass could be improved by removing the partly volatile matter of woody biomass before mixing with caking coal, and as a result, the possibility of using woody biomass as a raw material for coke-making with prior carbonization at temperatures of more than 500°C was found.
In recent years, the price of caking coal used in the production of metallurgical coke soars, and the price in 2012 was approximately twice that in 2005.1) It is expected that the prices will continue to increase in future. In addition, emissions of CO2 gas from the steel industry make up for approximately 15% of total emissions in Japan.2) Especially, CO2 emissions from the ironmaking process account for about 70% of all those from the steel industry, because large amounts of fossil fuels are used as both a heat source and a reducing agent in the process.3) Therefore, in relation to the need to ameliorate global warming, it is considered critical to reduce CO2 emissions from the ironmaking process, and to reduce coal consumption and find alternative sources. From this perspective, the recycling of the waste plastics in the coke-making process has been researched.4,5,6)
On the other hand, the use of woody biomass has also attracted attention as an alternative energy resource because it is carbon neutral. Woody chips, as byproducts of sawmills and the construction industry, are currently being used as fuel for power generation and so on. However, it is necessary to consider the utilization of forest remaining wood discarded from forest management processes such as tree thinning, as this forest remaining wood producing approximately 8 million tons each year are hardly used.7) Therefore, the utilization of woody biomass including high amounts of oxygen in the coke-making process has been the subject of research.8,9,10,11,12,13,14,15) Diez et al.11) found that eucalyptus sawdust and its carbonization products obtained at 415°C (tars and charcoal) inhibited the fluidity of coking coals to varying degrees. MacPhee et al.12) mainly studied the influence of charcoal particle size on the quality of the coke produced using charcoal additions. Coke made with a fine charcoal addition (–150 μm) was found to be considerably weaker than those made from a base blend, but an increase in the particle size of charcoal had a dramatic improvement on the quality of the coke produced. Additionally, the effect of biomass addition on the thermoplastic properties of coal has also been investigate,13,14,15) and it has been found that the fluidity of a coking blend is usually reduced by the addition of biomass, and that fluidity increased with an increase in the heating rate.
In this work, we conducted fundamental research on the possibility of using woody biomass as a raw material for the coke-making. Connectivity between coal particles in the coke with added woody biomass was evaluated quantitatively by observing the coke structure, and the strength was measured using an I-type tumbler tester for a single particle in order to investigate the effect of woody biomass addition on coke properties. Furthermore, a method for improving the influence of woody biomass addition on coke properties was found.
Caking coal (Goonyella coal: G), non-slightly-caking coal (White haven coal: W) and woody biomass (sawdust of Japanese cedar: B) were used in this study. Table 1 shows the properties of samples of coal and woody biomass. In addition, chars carbonized the sawdust of Japanese cedar at each temperatures (300, 400, 500 and 1000°C) in N2 gas atmosphere were also used as woody biomass char (BC(300), BC(400), BC(500) and BC(1000)). Table 2 shows the properties of those woody biomass char samples. Coals had a particle diameter of –1 mm, and woody biomass and chars had a particle diameter of –150 μm.
| Sample | Proximate analysis (wt%) | Ultimate analysis (wt%) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| a.r1) | dry basis | d.a.f.2) | |||||||
| Moisture | VM | FC | Ash | C | H | N | S | O3) | |
| Goonyella (G) | 1.4 | 24.1 | 67.3 | 8.6 | 87.40 | 5.20 | 1.90 | 0.60 | 4.90 |
| White haven (W) | 0.8 | 36.8 | 56.4 | 6.8 | 82.30 | 5.39 | 1.94 | 0.46 | 9.90 |
| Woody biomass (B) | 3.8 | 86.1 | 11.8 | 2.1 | 47.56 | 5.66 | 0.27 | – | 46.51 |
1) a.r: as received, 2) d.a.f.: dry ash free, 3) O: by diffrence
| Sample | Carbonization Temperature (°C) | Proximate analysis (wt%) | Ultimate analysis (wt%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| a.r1) | dry basis | d.a.f.2) | |||||||
| Moisture | VM | FC | Ash | C | H | N | O3) | ||
| BC(300) | 300 | 1.1 | 60.3 | 37.3 | 2.4 | 69.07 | 4.63 | 0.15 | 26.15 |
| BC(400) | 400 | 1.9 | 32.0 | 66.1 | 1.9 | 74.69 | 3.86 | 0.16 | 21.29 |
| BC(500) | 500 | 2.0 | 24.7 | 72.4 | 2.9 | 82.21 | 3.43 | 0.16 | 14.19 |
| BC(1000) | 1000 | 2.2 | 18.3 | 77.2 | 4.5 | 74.54 | 2.61 | 0.12 | 22.72 |
1) a.r: as received, 2) d.a.f.: dry ash free, 3) O: by diffrence
BC: Woody biomass char
Caking coal (G) and non-slightly-caking coal (W), woody biomass (B), or woody biomass char (BC(300), BC(400), BC(500) and BC(1000)) were mixed as shown in Tables 3 and 4. Samples were prepared using the designated mixing ratio based on the fixed carbon weight. The mixed sample was put into a ceramic crucible (test tube type with 11 mm inner diameter) so that the weight of fixed carbon in the mixed sample equaled approximately 0.4 g. A stainless steel rod (ϕ10 mm × L90 mm) was then inserted into the ceramic crucible to apply a pressure of about 6 kPa on the mixed sample. The ceramic crucible was put in a stainless steel sample holder as shown in Fig. 1. The sample holder was heated to 1000°C at a rate of 3°C/min in N2 gas atmosphere using a muffle furnace. The temperature was then retained at 1000°C for 60 min, and cooled to 200°C in the furnace. The coke sample obtained (carbonized sample) was buried in a resin, cut horizontally and then polished. Finally, a cross section of the polished coke sample was observed using an optical microscope.
| Fixed carbon basis (wt%) | Sample weight basis (wt%) | |||||
|---|---|---|---|---|---|---|
| Sample | G | W | B | G | W | B |
| G100 | 100 | – | – | 100.0 | – | – |
| G90–W10 | 90 | 10 | – | 88.7 | 11.3 | – |
| G80–W20 | 80 | 20 | – | 77.7 | 22.3 | – |
| G99.8–B0.2 | 99.8 | – | 0.2 | 99.0 | – | 1.0 |
| G95–B5 | 95 | – | 5 | 76.9 | – | 23.1 |
| G90–B10 | 90 | – | 10 | 61.2 | – | 38.8 |
G: Goonyella, W: White haven, B: Woody biomass
| Fixed carbon basis (wt%) | Sample weight basis (wt%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sample | G | BC (300) | BC (400) | BC (500) | BC (1000) | G | BC (300) | BC (400) | BC (500) | BC (1000) |
| G95–BC(300)5 | 95 | 5 | – | – | – | 91.3 | 8.7 | – | – | – |
| G95–BC(400)5 | 95 | – | 5 | – | – | 94.9 | – | 5.1 | – | – |
| G95–BC(500)5 | 95 | – | – | 5 | – | 95.3 | – | – | 4.7 | – |
| G95–BC(1000)5 | 95 | – | – | – | 5 | 95.6 | – | – | – | 4.4 |
G: Goonyella, BC: Woody biomass char

Schematic diagram of sample holder.
Connectivity between coal particles in the coke sample was evaluated by analyzing a macrostructure image of the cross section of a coke sample. Three macrostructure images were observed, and approximately 25% of the cross-sectional area of the coke sample was analyzed. There was hardly any variation in the porosity obtained from the image analysis, and the results of the image analysis were reliable. The coke structure derived from coal was distinguished from the other parts (the pore and coke structure derived from woody biomass), and the macrostructure image was binarized using image analysis software. Figure 2 shows an example of the macrostructure image binarized. From this, the area and number of coal particles connected within the coke structure derived from coal were determined (the white area in Fig. 2(b)). In this way, the connectivity between coal particles in the coke sample, how coal particles were connected during carbonization, was quantitatively evaluated.

Example of the macrostructure image binarized.
In order to quantitatively evaluate the influence of wood biomass addition on coke strength, the coke strength of single particles was measured using the I-type tumbler tester16) showed in Fig. 3. This tester is comprised of a stainless steel tubular vessel with 40 mm inner diameter and a length of 350 mm length, a rotation speed-setting device, and a motor. A single particle from a coke sample was used in the strength measurement, and the procedure was repeated twice. The rotation speed was set to 30 rpm. The stainless steel tubular vessel was turned at the rotation speed of 30 rpm for 25 min. The sample in the tubular vessel was extracted every five minutes, and samples with particle sizes of more than 1 mm were weighed. After that, all sample particles were put in the tubular vessel and it was turned again. The tumbler strength index was defined to indicate the coke strength quantitatively, as described in Eq. (1).
| (1) |

Schematic diagram of the I-type tumbler tester.
Figure 4 shows macrostructure images of G100, G90–W10 and G80–W20. The bright-gray part, dark-gray part, and black part are the coke structure derived from coal, resin, and pore, respectively. When White haven coal (non-slightly-caking coal) was added to Goonyella coal (caking coal), it was possible to observe coal particles, such as those of a raw coal, that did not soften and melt during carbonization. The number of these coal particles increased with an increase in the mixing ratio of non-slightly-caking coal.
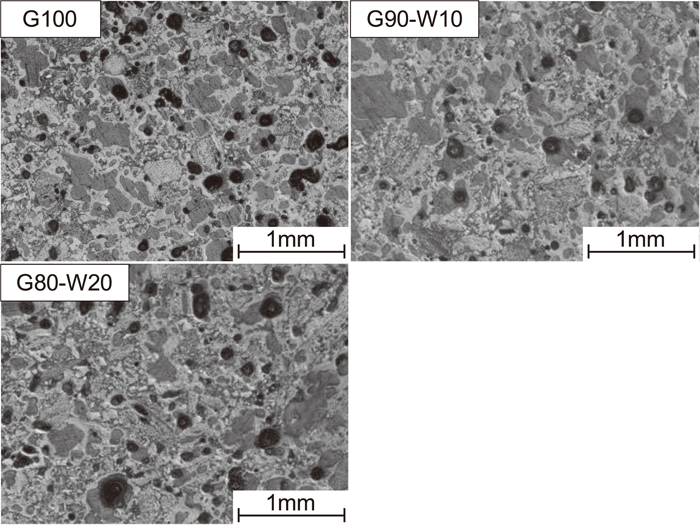
Macrostructure images of G100, G90–W10 and G80–W20.
Figure 5 shows macrostructure images of G99.8–B0.2, G95–B5 and G90–B10. The dark-gray part is resin and woody biomass, and the other parts are the same as those in Fig. 4. In the case of G95–B5 and G90–B10, the caking coal particles exist as a single particle without connection to other particles; however the particles soften, melt, and swell during carbonization. Moreover, with an increase in the mixing ratio of woody biomass, there is an increase in the distance between coal particles, as a substantial amount of carbonaceous matter derived from woody biomass exists between the coal particles. In the case of G99.8–B0.2, where the mixing ratio of woody biomass is very small, the coke structure that was similar to that of G100 in Fig. 4 was observed.

Macrostructure images of G99.8–B0.2, G95–B5 and G90–B10.
The area fraction of coal particles obtained by image analysis is shown in Fig. 6. The term “Others” in Fig. 6 indicates the pore and the coke structure derived from woody biomass. In comparison with G100, the area fraction of the particle (lump) with an area less than 0.5 mm2 increased, and that with an area greater than 0.5 mm2 decreased when non-slightly-caking coal was added to caking coal. Accordingly, the connectivity between coal particles in the coke sample decreased with addition of non-slightly-caking coal to caking coal. On the other hand, when woody biomass was added to caking coal (G95–B5 and G90–B10), there were no particles with areas larger than 0.5 mm2, and the area fraction of particles with areas less than 0.5 mm2 increased considerably. This result suggests that connectivity between coal particles in the coke sample drastically decreases when woody biomass is added to caking coal.

Effect of the addition of non-slightly-caking coal and woody biomass on the area fraction of coal particles.
Figure 7 shows the weight change curve of the woody biomass, heated at a rate of 3°C/min in N2 gas atmosphere, obtained using thermogravimetric analysis. Drastic weight loss occurred at temperature between 250 and 350°C due to the release of volatile matter from the woody biomass, and it is supposed that the wood biomass shrinks with release of volatile matter. In the case of woody biomass addition, a large number of woody biomass particles exist between the coal particles, so the density of woody biomass is much smaller than that of coal. Therefore, voids are formed between coal particles during carbonization because woody biomass begins to release volatile matter and shrink at a lower temperature (250–350°C) than a softening and melting temperature (410–490°C)17) of Goonyella coal (caking coal). After that, when caking coal swells at a softening and melting temperature, it cannot fill the void between coal particles. This is because there are many voids between the coal particles. Even if the void between coal particles can be filled, the swelling of Goonyella coal is in a state of free expansion, and the consolidation effect becomes small. Moreover, in the case of plastic addition to coal, the coal caking property is inhibited by a stabilization of a radical derived from plastic.5) In addition, heteroatoms like oxygen have a deleterious effect on the thermoplastic properties of coal.18) From these, volatile matter released from woody biomass (including high amounts of oxygen) may inhibit the thermoplastic properties of coal. That is why the adhesion between coal particles, and between coal particle and woody biomass particle, is insufficient, as shown in Fig. 8. Where there is a small mixing ratio of woody biomass (G99.8–B0.2), the area fraction distribution of G99.8–B0.2 is similar to that of G100, as it is difficult to inhibit the adhesion between coal particles. Although the area fraction of the particle with an area greater than 0.5 mm2 decreases slightly, that reason is that the woody biomass particles control the further growth of the coke structure that is connected by coal particles.

Weight change curve of woody biomass heated at a rate of 3°C/min in N2 gas atmosphere.

Microstructure image of G95–B5.
As mentioned above, the release of volatile matter from woody biomass at low temperatures during carbonization is one of the factors that inhibits connectivity between coal particles. Accordingly, woody biomass was heated at each temperature (300, 400, 500 and 1000°C) in a N2 gas atmosphere for 60 min, and woody biomass char with partly removed volatile matter was prepared. The effect of removing the volatile matter from the woody biomass and the carbonization temperature on the connectivity between coal particles in the coke sample was investigated. Macrostructure images of G95–BC(300)5, G95–BC(400)5, G95–BC(500)5 and G95–BC(1000)5 are shown in Fig. 9. The rugged dark-gray part is the woody biomass char, and the other parts are the same as those shown in Fig. 5. Unlike the coke structure in the coke sample with added woody biomass shown in Fig. 5 (G95–B5, G90–B10), coal particles were seen to be connected when woody biomass char was added to caking coal.

Macrostructure images of G95–BC(300)5, G95–BC(400)5, G95–BC(500)5 and G95–BC(1000)5.
The area fraction of coal particles obtained from macrostructure images in Fig. 9 is shown in Fig. 10, with results of G100 and G95–B5. For the samples with woody biomass char addition, in comparison to the sample with woody biomass addition (G95–B5), there is a small decrease in the area fraction of particles with areas less than 0.5 mm2, and particles with areas larger than 0.5 mm2 are in existence. Moreover, the area fraction of particles with areas greater than 0.5 mm2 increases with an increase in the carbonization temperature of woody biomass char. Figure 11 shows microstructure image of G95–BC(1000)5. When woody biomass char with partly removed volatile matter is added, woody biomass char adheres to the coal particles that softened and melted, and exists in the coke structure (carbonaceous matters) derived from coal, unlike in the microstructure of G95–B5, as shown in Fig. 7. These results indicate that the inhibition of connectivity between coal particles during carbonization, due to the release of volatile matter from woody biomass, can be reduced by partly removing the volatile matter in advance. Furthermore, the effect is larger with an increase in the carbonization temperature of woody biomass char.
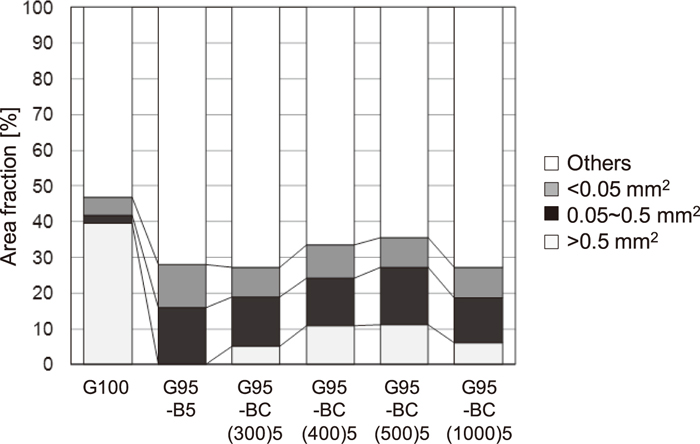
Effect of woody biomass or woody biomass char addition on the area fraction of coal particles.

Microstructure image of G95–BC(1000)5.
Figure 12 shows changes in TI+1 mm of G100, G90–W10, G80–W20, G99.8–B0.2, G95–B5, and G90–B10 with time. TI+1 mm of G100 was 92.0%, and TI+1 mm of G90–W10 and G80–W20 (with addition of non-slightly-caking coal) were 89.3% and 87.5%, respectively. The coke strength of the coke sample deteriorates with an increase in the mixing ratio of non-slightly-caking coal. In the case of woody biomass addition, on the other hand, TI+1 mm of G95–B5 and G90–B10 decreased substantially to 6.2% and 3.3%, respectively, in comparison with TI+1 mm of G100. These results indicate that the addition of woody biomass causes a remarkable deterioration in the strength of coke. The reason is that connectivity between the coal particles during carbonization is inhibited by the release of volatile matter from woody biomass, as mentioned in Sec. 3.1. However, TI+1 mm (96.3%) of G99.8–B0.2 was roughly equivalent to TI+1 mm of G100. Therefore, the addition of a small amount of woody biomass weighing 0.2 wt% on the basis of the fixed carbon weight (1 wt% on the basis of the sample weight) does not affect coke strength.

Changes in TI+1 mm with time of G100, G90–W10, G80–W20, G99.8–B0.2, G95–B5, and G90–B10.
Figure 13 shows changes in TI+1 mm of G95–BC(300)5, G95–BC(400)5, G95–BC(500)5, and G95–BC(1000)5 with time. TI+1 mm (7.4%) of G95–BC(300)5 was about the same as TI+1 mm of G95–B5. There was a remarkable increase in TI+1 mm of G95–BC(400)5 to 73.7%, and TI+1 mm of G95–BC(500)5 and G95–BC(1000)5 reached 85.2% and 82.6%, respectively. Values of TI+1 mm increase with an increase in the carbonization temperature of woody biomass char. TI+1 mm of G95–BC(500)5 had almost the same value as that of G95–BC(1000)5. This indicates that the shrinkage of wood biomass after resolidification of the caking coal (at 500°C and above) has very little influence on the strength of coke. From these results, it is suggested that the carbonization of woody biomass at temperatures of more than 500°C reduces the inhibition of connectivity between coal particles during carbonization due to the release of volatile matter from woody biomass, and improves coke strength to the same degree as that of the coke sample made from caking coal.

Changes in TI+1 mm with time of G95–BC(300)5, G95–BC(400)5, G95–BC(500)5, and G95–BC(1000)5.
Connectivity between coal particles and the coke strength of a coke sample to which non-slightly-caking coal, woody biomass, and woody biomass char were added to caking coal, were evaluated quantitatively by observing the coke structure and using an I-type tumbler tester for single particles, in order to investigate the possibility of using woody biomass as a raw material in coke-making. The following results were obtained.
(1) Connectivity between coal particles during carbonization was inhibited by the addition of non-slightly-caking coal to caking coal. This inhibition of connectivity between coal particles caused a decrease in coke strength.
(2) When woody biomass was added to caking coal, connectivity between coal particles during carbonization, and the coke strength, drastically decreased, although the addition of a small amount of woody biomass (0.2 wt% on the basis of the fixed carbon weight) hardly affected either the connectivity between the coal particles or the coke strength.
(3) The inhibition of connectivity between coal particles in the coke sample with added woody biomass can be reduced by partly-removing the volatile matter of woody biomass in advance. The effect of this was larger with an increase in the carbonization temperature of woody biomass char.
(4) The carbonization of woody biomass at temperatures of more than 500°C improved the coke strength of the coke sample with added woody biomass char to the same degree as that of the coke sample made from caking coal.
This work has performed in a Research Group for Progress in Cokemaking Technology for Low-quality Coals and Unused Carbon Resources, in The Iron and Steel Institute of Japan (the chief examiner is Prof. H. Aoki, from Tohoku Univ.). The authors would like to acknowledge the contribution of all research group members.