2014 Volume 54 Issue 12 Pages 2687-2696
2014 Volume 54 Issue 12 Pages 2687-2696
Self-reduction experiments of mill scale with four potential carbonaceous reductants (charcoal, coal char, blast furnace coke and petroleum coke) were carried out by thermogravimetry (TG) in order to choose one allowing the highest reduction rate and overall conversion. The fastest rate of self-reduction was measured for the charcoal and the slowest for the petroleum coke. The Friedman method of kinetics data analysis was used to calculate apparent activation energy at different conversion rates. Based on these values it can be concluded that the Boudouard reaction controls the rate of self-reduction with charcoal up to about 60% of conversion and even more for others reductants. It has also been demonstrated that the morphology of the iron produced strongly depends on reactivity of carbonaceous reductant.
In the steelmaking processes, about 500 kg of solid wastes of different nature (including blast furnace (BF) and basic oxygen furnace (BOF) slag) per ton of steel are generated.1) A big part of that is already recycled but the new, more efficient ways of recycling are still explored. One of the wastes generated in steel plants is the scale which represents about 2% of the steel produced. It is formed during the continuous casting and rolling mill processes when steel is subject to thermal gradients in oxidant atmospheres, which promotes the growth of iron oxides layer at the surface of steel.2) Such a scale is very rich in iron and generally contains three iron oxides - wüstite, magnetite and hematite.3)
In the integrated steel plants, the mill scale is habitually used as raw material at the sintering plants but the recycling of this waste has also been reported in the form of briquettes used in BOF steelmaking.4,5,6) In the case of mini-mills which operate using electric arc furnaces (EAF) and where there are no reduction reactors, such as a blast furnace, the scale recycling is more difficult and this waste is often sent to landfills or used in the cement industry. Some authors have already reported the possibility of recycling mill scale in mini-mill plants through the introduction of self-reducing briquettes in EAF, but the details are still being investigated.7,8,9)
The EAF process operates at a lower charge capacity and its time-to-smelt is shorter, in comparison with the reduction reactors. While the reduction reactors require high-strength self-reducing briquettes to achieve high performance, in the EAF process kinetic phenomena seem to be the most important parameter. If the iron oxides are not reduced before charge melting, it may lead to an increase in the energy consumption and a decrease in the metal yield of the process. So, to make the use of self-reducing briquettes viable in an EAF, such agglomerate must have a high kinetics of reduction of the iron oxides content.
The self-reduction (carbothermal reduction) technology consists essentially of using the agglomerates with an intimate mixture between the iron oxides and the reducing carbonaceous agent, the quantity of which must be sufficient to reduce completely the oxides content. The main chemical reactions resulting from heating such composites are the coupled gaseous reduction of iron oxides (1) and gasification of carbon or Boudouard reaction (2):
| (1) |
| (2) |
As these reactions occur in series, one or the other can control the overall reaction rate in the self-reducing composites depending on a variety of experimental conditions such as the composition (oxide to carbon ratio), form (pellet, briquette, packed bed) and size of agglomerate, the heating regimes and the surrounding atmosphere composition and pressure. As reviewed by Donskoi et al.,10) a vast majority of researchers claim the Boudouard reaction as a limiting step of a self-reduction process.11,12,13,14,15) Others propose a mixed control regime with the influence of both reactions (gasification and reduction)or a mixed gasification and heat transfer control regime.16,17,18,19,20) Pure heat transfer control has also been postulated and modelled.21)
These findings are based on different experimental approaches such as the continuous weight loss of composite sample, the reactor output gas analysis and the temperature profiles inside the composite piece. The influence of the constituent particles nature and size of iron oxide, carbonaceous materials and some additives on the kinetics of overall reaction and the magnitude of its apparent activation energy are often used to identify a rate controlling step. The relatively high activation energy values (250–350 kJ/mol) are taken as being characteristic for the Boudouard reaction control while the lower values indicate an increasing role of reduction in overall self-reduction rate control.10,14) According to Iguchi et al.22,23,24) the carburization of freshly reduced iron by direct carbon dissolution C(s) = [C] is another important reaction which could interfere in self-reduction, especially at the reduced pressures and high temperatures.
Due to the importance of the Boudouard reaction and regarding several possible reducing agents for use in such agglomerates, different sources of carbon such as biomass char, coal, coal char, lignite, metallurgical and petroleum cokes, waste plastics as well as graphite (in some fundamental studies) have been tested.10,22,23,24,25,26,27) On the other hand, the hematite and magnetite ores, synthetic wustite as well as some waste materials (EAF dust, mill scale) were used as the iron oxide bearing materials in the self-reducing mixtures and agglomerates.10,11)
In addition to the reaction rate limits, another important issue is the mechanical strength of the agglomerates during the reduction. It depends to a large extent on the morphology of the resulting iron, which can lead to some adverse effects such as swelling and cracking of agglomerate. The nature of the reductant and temperature profile of self-reduction influence these phenomena.28,29,30)
The mechanism and kinetics of self-reduction has already been modelled to better understand the rate phenomena and to optimize the iron oxide/carbon composite technology by better selection of constituents and their processing conditions.10,18,19,20,21,24) These models take into consideration not only the kinetics of gasification and reduction reactions but also the mass and heat transfer phenomena and balances. They need precise measures of the involved reactions rate constants and some structural parameters (porosity, specific surface, etc.) which influence the chemical reactions and transport phenomena. The heat transfer is of particular interest due to strongly endothermic character of Boudouard reaction, it could become the rate-limiting step.21,29)
To find the right way to introduce the self-reducing briquettes in the EAF, it is crucial to evaluate the characteristics of the raw materials to predict its self-reduction performance under such conditions. Bearing this in mind, the scale used in this work was first characterized and its reduction behaviour in pure CO and CO–CO2 mixtures was investigated.3) In the next step, which was the goal of this work, four carbonaceous materials was evaluated as the potential scale reducing agents for the self-reducing briquettes to use in the EAFs. For that gasification kinetics of each reductant by pure carbon dioxide was first measured and next the self-reduction tests were performed in order to determine the rate and controlling steps of process. Moreover, the influence of reductant on the morphology of produced iron was investigated to better understand the mechanism of self-reduction.
The scale used in this work was provided by a mini-mill plant. It was sampled and prepared according to the proportion generated in the continuous casting and rolling mill. The chemical composition of the scale is presented in Table 1(a) and the phase analysis by Mössbauer spectroscopy is given in Table 1(b). The content of reducible oxygen in the scale, calculated from both analysis and confirmed by gaseous reduction of scale, is 22.3 mass%.3)
| Elements | Fe | Al | W | Mn | Cr | Ni | Si |
|---|---|---|---|---|---|---|---|
| mass% | 69,0 | 1,95 | 0,83 | 0,65 | 0,32 | 0,10 | 0,05 |
| Iron phases | Fe2O3 | Fe3O4 | FeO | Fe |
|---|---|---|---|---|
| mass% | 7 | 18 | 74 | 1 |
As received, the scale is a mixture of coarse particles, mainly having a shape of platelets (20 mass% above 2 mm) and fines (20 mass% under 0.3 mm). As it is shown in Fig. 1(a) the coarse particles exhibit layered microstructure with three distinct zones. The outer layer is relatively thin and porous. It is mainly composed of hematite and magnetite. The intermediate layer is made of dense, columnar grains of wüstite. The inner layer is a very porous wüstite. For self-reduction tests the scale was crushed and the fraction passing the 100 μm sieve was used to prepare the mixtures with the carbonaceous reductants. Its particles size distribution was measured by size and shape analysis system QICPIC (Sympatec GmbH). It is shown in Fig. 2(a).

Micrograph showing the morphology of scale: a) cross-section of scale platelet, b) coarse particles of powdered scale.

Particles size distribution (a) and shape factor - sphericity versus particles size (b) measured by image analysis.
Four carbonaceous materials were studied as the potential reducing agents: charcoal fines (Charcoal), char from bituminous coal (Coal char), blast furnace coke (BF coke) and petroleum coke (Petcoke). The proximate and ultimate analyses of these materials are presented in Table 2. For the gasification and reduction tests they were crushed, when necessary, and the fraction passing the 160 μm sieve was used. The particles size distribution of the reductants was also measured by QICPIC analysis system and it is plotted in Fig. 2(a) together with the scale particles size distribution curve. It is interesting to note that the particles size measured by image analysis exceeds the maximum size expected from screening (100 μm for scale and 160 μm for reductants). This is especially true for the scale. It could be explained by the shape of particles. Using QICPIC software the different shape factors could be calculated. In Fig. 2(b) is presented the sphericity factor vs. size of particles for all materials used in this study. It shows that the particles of powdered scale (especially the bigger ones, >100 μm) have less spherical, but more elongated shape then carbonaceous materials. This elongated shape of scale particles, seen also at the micrograph in Fig. 1(b), results from the cleavage of layered structure of the parent scale platelets.3)
| Sample | Proximate analysis (mass%) db | Ultimate analysis (mass%) daf | ||||||
|---|---|---|---|---|---|---|---|---|
| Ash | Volatile Matter | Fixed Carbon | C | H | N | S | O | |
| Charcoal | 2.0 | 20.1 | 77.8 | 85.8 | 3.17 | 1.07 | 0.08 | 9.97 |
| Coal char | 11.3 | 4.2 | 84.5 | 89.3 | 3.75 | 1.58 | 1.01 | 4.37 |
| BF coke | 10.5 | 1.1 | 88.5 | 96.2 | 0.54 | 1.23 | 0.78 | 1.25 |
| Petcoke | 0.9 | 7.5 | 91.6 | 92.8 | 3.25 | 2.42 | 0.79 | 0.69 |
db – dry basis, daf – dry and ash free basis
The reactivity of all carbonaceous materials towards carbon dioxide was first measured by Netzsch STA 409 PC Luxx thermobalance using the procedure with stepwise temperature increase, established in the previous studies on coke reactivity.32,33) A sample of powdered reductant with mass about 50 mg was poured into an alumina crucible of 6 mm in inner diameter and 5 mm in height. The sample was first heated up to 1100°C under nitrogen and kept at this temperature for 10 minutes in order to release the residual volatile matters. Then, the temperature was lowered to 800°C. After that nitrogen was replaced by carbon dioxide (300 ml/min) and a set of reaction rate measurements followed. The sample was kept at 800, 850, 900, 950, 1000, 1050 and 1100°C successively for different periods of time (ranging from 60 to 10 minutes), sufficient to observe stable gasification rate at each temperature plateau. This procedure limits number of experiments needed to measure the influence of temperature on gasification rate. It is worth to note that care was taken to not exceed 20% of cumulated sample fractional mass loss in order to prevent the influence of fractional gasification on the gasification rate as it was well demonstrated by Turkdogan and Vinters.31)
2.3. Self-reduction KineticsThese tests were carried out to verify the kinetic behaviour of self-reducing mixtures with different reducing agents and scale as the source of iron oxides. To avoid the influence of residual volatiles on the reduction, all carbonaceous reducing agents were first heated under nitrogen up to 1100°C and then, after soaking during 10 minutes, cooled down. Such devolatilised reductants were blended with scale at the reductant/scale ratio of 1/4 (by weight), giving a C/O molar ratio of about 3/2. The sample of self-reducing mixture with mass about 200–400 mg was poured into an alumina crucible having the same inner diameter as for gasification (6 mm) but higher (10 mm). Figure 3 shows the polished cross-section of the crucible with the mixture of BF coke and scale after about 50% of fractional mass loss. The bulk density of the samples inside the crucible was 0.3–0.4 g/cc for reducing agents and 1.2–1.3 g/cc for self-reducing mixtures. The self-reduction kinetics measurements were carried out under non-isothermal conditions using the same Netzsch thermobalance as in the gasification tests. In the first series of study, the self-reduction kinetics was measured at a heating rate of 20 K/min. However, to better assess the apparent activation energy of self-reduction, in the second series, for two of them (charcoal and BF coke), the reduction rate was measured at a different heating rate (ranging from 3 to 40 K/min).

Cross-section of alumina crucible with the mixture of scale and BF coke after partial reduction showing the crucible size and shape as well as the main sample characteristics for self-reduction tests (a) and gasification tests (b).
In order to have a better insight into the self-reduction mechanism, the microscopic examination of partially reduced samples has been performed with a metallographic optical microscope (Reichert-Jung MeF3) on polished samples (mounted on epoxy resin).
Figure 4 explains a typical thermogravimetric curve for the gasification of reductant (BF coke) in pure CO2. The weight of the sample decreases slightly during heating in nitrogen, due to the release of residual volatile matters and becomes constant after 10 minutes at 1100°C. Next, during the sample cooling to first isothermal plateau (here 900°C) and after the gas shifting from N2 to CO2 some artefacts affect the mass loss curve. However, after few minutes the temperature and mass signals are stabilised and stepwise gasification is going on. The slope of the mass loss curve
| (3) |

Typical thermogravimetric curve for the gasification of BF coke sample in pure CO2 at step by step increasing temperature.
Figure 4 shows that the mass loss increased during each isothermal step which intervals were shortened from 30 min at 900°C to 10 min at 1100°C. The fractional gasification calculated from the mass loss at these steps was relatively small and changed from 2 to 5%. Consequently, the cumulated fractional gasification during the whole test with BF coke, presented at Fig. 4 as an example, reached only 18%. For more reactive materials, the time of isothermal steps at high temperature was even shorter (around 5 min) in order to not exceed 20% of cumulated fractional gasification.
The gasification rates of four carbonaceous materials in pure CO2 at the temperature range from 800 to 1100°C are presented in Fig. 5 as an Arrhenius type plot. It shows that the gasification rate of charcoal is the highest. It is followed by char from bituminous coal. The BF coke and petroleum coke have the lowest reactivity towards CO2 in the range of the temperatures investigated. Such variation of reactivity among these materials is quite justified by the carbon microstructural differences, which are well characterized by their textures shown in Fig. 6. The petroleum coke and the BF coke reveal very anisotropic texture, which is considered to be less reactive. The char from bituminous coal exhibits mainly isotropic texture considered to be very reactive. The micrograph of charcoal shows the isotropic texture and the high porosity, which certainly contribute together to its highest reactivity.

Gasification rates in pure CO2 as a function of temperature for the studied carbonaceous materials.

Typical textures of four carbonaceous reductants: a) charcoal - porous and isotropic, b) coal char – isotropic, c) BF coke – anisotropic, d) petroleum coke – anisotropic.
The mean apparent activation energy for the gasification in pure CO2 was calculated from the slope of the regression lines for whole temperature range covered by the kinetic data of each carbonaceous material. It varies from 228 to 272 kJ/mol and is within the large range of values reported in literature11,12,14,17,19,31,35,40) as characteristic for the Boudouard reaction.
However, in the conditions of TG measurements (crucible size and form, sample mass and volume, particle size distribution, gas composition and flow rate) some influence of mass transfer phenomena (especially inter-particles diffusion inside the crucible) cannot be completely excluded. Recently, Malekshahian et al.35) studied the mass transfer limitation in TGA experiments of carbon materials gasification. The experimental and calculation results of this study showed that the initial mass and particle size of sample influenced the reaction rate obtained by TG analysis. The authors used the effectiveness factors to account the difference between gasification rate measured in TGA experiment and calculated intrinsic gasification rate. They found that for the petroleum coke this difference was relatively small, increasing from 1% to around 10% in the temperature range 925–1000°C. It is interesting to note that activation energy of intrinsic gasification rate constant of petroleum coke calculated from Malekshahian’s data35) is 280 kJ/mol, which is very close to the apparent activation energy obtained in our TG gasification tests (272 kJ/mol in Fig. 5). This validates the conditions of our TG tests showing that they assure the chemical reaction control at least for the low reactive reductants, like the petcoke.
However, for high reactive reductants, like coal char and charcoal, some influence of mass transfer limitations cannot be excluded. In the same study35) it was found that, measured in the similar conditions at 950°C, the gasification rate constant of the highly reactive activated carbon was only about a half of the calculated intrinsic rate constant. According to the authors, it is due to the effects of sample mass which influences the external and inter-particle diffusion limitations and the carbon particle size which changes intraparticle diffusion limitation. In our TG experiments, the similar effects couldn’t be completely eliminated by the choice of experimental conditions. It is attested by the decrease of apparent activation energy with temperature of the two most reactive reductants. In fact, for the charcoal the apparent activation energy of gasification in the temperature range 800–900°C is about 280 kJ/mol but in the range 900–1000°C it attains just 160 kJ/mol. Such decrease of apparent activation energy with temperature is lower for the coal char but it is still significant (260 kJ/mol for 800–1000°C and 190 kJ/mol for 1000–1100°C), indicating some role of interparticle diffusion limitation. Nevertheless, in Fig. 5, for simplicity, only the mean values of apparent activation energy of gasification are shown because, even more or less influenced by mass transfer limitations, they are noticeably higher than apparent activation energy of reduction with which they are further compared in order to find the reaction which influences more the self-reduction rate.
3.2. Self-reduction Rates with Different ReductantsFigure 7 shows the mass loss curves obtained with the different self-reducing mixtures during the non-isothermal runs at a heating rate of 20 K/min up to 1100°C. For the sample of scale mixed with charcoal, the reduction started at about 850°C and was very fast. Consequently, it was completed before the sample reached 1100°C. The reduction rate of scale mixed with the char from bituminous coal was close to that measured with charcoal, but somewhat slower. On the other hand, the mass loss of the samples containing blast furnace and petroleum coke started at a higher temperature (about 950°C and 1000°C, respectively) and the reduction rate was much slower, being however higher for the blast furnace coke than for the petroleum coke. It is thus worth to note that the self-reduction rate by these four reductants corresponds quite well to their rate of gasification in pure CO2, where charcoal was the most reactive, followed by coal char, BF coke and petcoke. A similar ranking of reductants was reported recently by Murakami and Kasai26) who studied the reduction mechanism of iron oxide-carbon composite with reactive char obtained by pyrolysis of polyethylene and compared it with other carbonaceous reductants (charcoal, metallurgical coke and graphite).
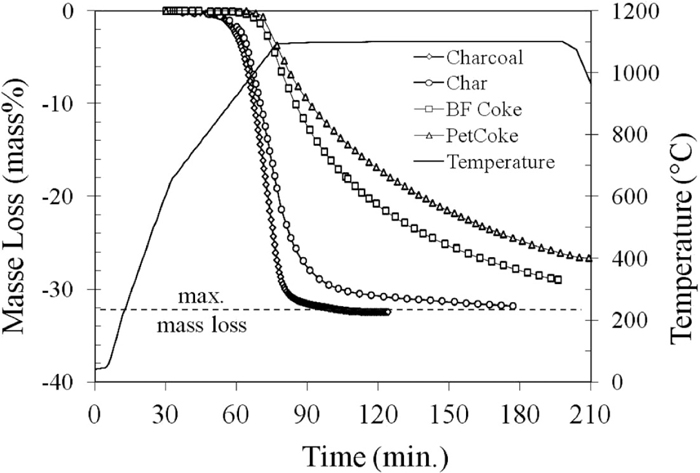
Mass loss curves of four self-reducing mixtures during the heating up to 1100°C.
To get a better insight into the self-reduction mechanism and the limiting reaction step, a non-isothermal series of TG measurements at different heating rates were carried out for two reductants: the high reactive charcoal and the low reactive blast furnace coke.
Figures 8 and 9 show the curves of mass loss versus time obtained in these series respectively with charcoal and BF coke as a reductant. Both figures reveal that self-reduction is completed when the relative mass loss reaches about 32 mass%. Consequently, this maximum fractional mass loss was used to calculate the conversion α defined as
| (4) |
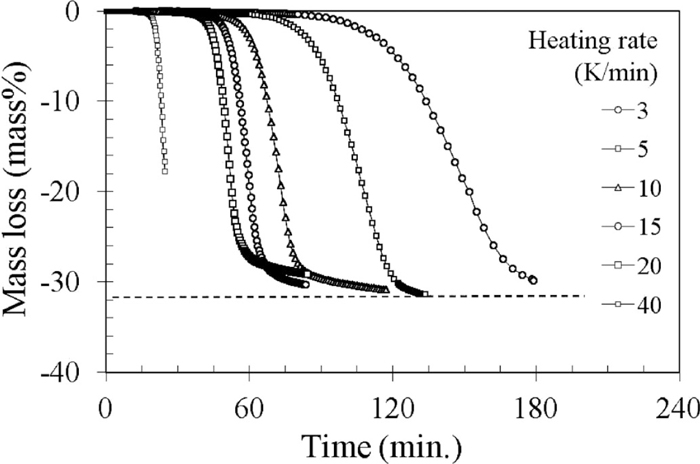
The mass loss curves for different heating rates with charcoal as reductant.

The mass loss curves for different heating rates with BF coke as reductant.
Figures 10 and 11 show the mass loss curves from Figs. 8 and 9 transformed into the conversions versus temperature curves, but just for the non-isothermal period. These figures demonstrate that for the chosen TG conditions (temperature maximum 1100°C for charcoal and 1200°C for BF coke) it was not always possible to complete reaction below the maximum test temperature (during non-isothermal period), especially in the runs with high heating rate. From the kinetic curves obtained with different heating rate (dT/dt), according to well known Friedman method,34) the conversion rate (dα/dt) at different conversion α was calculated and plotted on the Arrhenius type diagrams presented in Figs. 12 and 13. From these plots the apparent activation energy of self-reduction reaction at different conversion degrees were calculated.

Conversion versus temperature for different heating rates with charcoal as reductant.

Conversion versus temperature for different heating rates with BF coke as reductant.

Friedman plot of self-reduction data with charcoal as reductant.

Friedman plot of self-reducing data with BF coke as reductant.
It is seen at these figures that the apparent activation energy at the low conversions (< 0.6) is relatively high. It ranges from 370 to 280 kJ/mol and corresponds well to the values reported as characteristic for carbon gasification by CO2 under a purely chemical control.14,17,31,35,40) These values are even slightly higher than those obtained from the gasification tests with pure carbon dioxide (Fig. 5). Therefore, they suggest that in the self-reducing mixtures the gasification of carbon by CO2 restored via indirect reduction of wustite occurs without external and interparticle diffusion limitation due to the intimate contact of reactants. This is especially true at low conversion (α =0.1) which was reached in the relatively low temperatures (850–950°C with charcoal and 1000–1050°C with BF coke). It is also worth to note here that the reduced value of apparent activation energy of charcoal gasification by pure CO2 in the high temperature range (160 kJ/mol) is quite a one-half of 320 kJ/mol measured in self-reduction test. Such a relation (Ekinet/2 = Ediff is considered, since the fundamental works of Walker et al. (in Laurendeau40)) on the porous carbon gasification, as the clear sign that the gasification is controlled by diffusion.
Furthermore, when charcoal is used as reductant (Fig. 12), the progressive decrease of apparent activation energy with increasing conversion (and temperature) from 320 kJ/mol at α =0.1 to 115 kJ/mol at α =0.9 is observed. Unfortunately, the last value is a very rough estimation from only three points of data. Nevertheless, it fits well with trend of the activation energy diminution apparent already at lower conversion which can be explained by the increasing role of reduction in the overall self-reduction rate. In fact, the rate of iron oxides reduction decreases with fractional reduction and it also should be true for the scale used in this study. The activation energy of the fine iron oxide reduction by CO reported by Moon and Sahajwalla varie from 36 to 75 kJ/mol.14) Fortini and Fruehan17) estimate the activation energy of wustite reduction by CO as high as 168 kJ/mol which is a bit higher then upper limit of the 60–150 kJ/mol range given by Coetze et al.19) In our previous work3) on the reduction of the scale by CO, the activation energy of reaction rate constant was found to be about 80 kJ/mol. It was also observed that the gaseous reduction of scale occurs according to the shrinking core model under chemical control, i.e. the reduction rate decreases with fractional reduction F proportionally to (1–F)2/3. It means that in the self-reduction process the reaction of reduction slows down when the total conversion increases and thus, progressively, it can become a limitation for the overall self-reduction rate. This is especially true for high reactivity reductant (like charcoal), which reacts sufficiently fast in the temperature range where the reduction rate of iron oxide is relatively low due to its feeble initial reducibility or it becomes low when fractional reduction increases. In the non-isothermal self-reduction with BF coke presented at Fig. 13 such clear decrease of apparent activation energy doesn’t appear. That could be explained by the relatively low maximum conversion (0.6) for which the energy could be calculated due to the available kinetics data limited by the maximal temperature in our TG measurements (1200°C). However, it is seen from this series of data that, like for the charcoal, the apparent activation energy of self-reduction is, in the large range of conversion, clearly higher than that which was measured for pure gasification of BF coke by CO2.
The role of reduction as a partially limiting reaction in self-reduction, which appears especially in the kinetics series with charcoal, is supported by some previous works on the self-reduction of different iron oxides by different carbon materials. Fortini and Fruhan17,18) analysing the reduction of wustite/carbon composites claim that reduction of wustite has a significant effect on the overall rate of self-reduction at the temperature above 1086°C. The conclusions of Srinivassan and Lahiri16) go even further because they assert that the decrease of apparent activation energy from about 400 kJ/mol at the beginning (20% of reduction) to 56 kJ/mol at the late stage of reaction (80% of fractional reduction) proves that the reaction limiting overall self-reduction rate changes from the carbon gasification to the reduction of wustite. On the other hand, Moon and Sahajwalla14) analysing the variation of the apparent activation energy of self-reduction with the reductants of different reactivity (due to its nature or changed by catalyst addition) come to the conclusion that even in moderate temperature of 1000°C the increase of reductant reactivity can decrease the limiting role of gasification. Thus, the self-reduction rate can shift from the Boudouard reaction control to the mixed Boudouard reaction/wustite reduction control regime.
Figure 14 resumes the results of kinetic study, specifically the effect of reductants reactivity and iron oxides reducibility on the controlling steps of self-reduction in non-isothermal conditions (progressive temperature increase). During a heating, the self-reduction starts when carbon gasification allows a regeneration of carbon oxide and thus maintaining a sufficient reduction potential inside the self-reducing mixture. When a highly reactive reductant (like charcoal) is used this happens at about 800°C. However, with a low reactivity reductant (like BF coke or Petcoke) this arrives at about 950°C or more. Nevertheless, in this temperature range (800–1000°C) the gasification rate is still lower than the possible gaseous (indirect) reduction rates and by consequence it controls the overall rate of self-reduction. At higher temperatures, above 1100°C, it is possible that the self-reduction shifts from the gasification control regime to a mixed gasification/reduction or even a pure reduction control regime. This is possible firstly due to the acceleration of Boudouard reaction, owing to its significantly higher activation energy, which varies from 200 to 400 kJ/mol while wustite reduction activation energy is in the range from 60 to 150 kJ/mol. Secondly, it is possible due to the relative slow down of reduction rate when reduction degree increases. Thus, the reaction control regime establishes not only the overall self-reduction rate but it also sets up a reduction gas composition within the self-reducing mixture. This composition approaches wustite/iron equilibrium when gasification is the controlling step or moves close to Boudourd reaction equilibrium when reduction controls overall self-reduction rate as it was experimentally showed by Moon and Sahajvalla.14)

Schematic representation of the reactions rate control regimes in self-reduction mixtures with the reductants of different reactivity. (the activation energy range for the gasification reaction (*) according to Refs. 11), 14), 17), 19), 31), 35), 40) and for the gaseous reduction of iron oxides (**) as reviewed in Refs. 14), 17), 19)).
The rate limiting factors that we depict in Fig. 14 are based on our study of self-reduction in the temperature range 800–1100°C. However, at higher temperature (1100°C and above) additional reactions and physical phenomena could interfere in the self-reduction mechanism modifying the reaction kinetics and the reduced iron morphology as showed Iguchi at all.22,23) These reactions are the carburization of freshly reduced iron by solid carbon dissolution: C = [C] and the direct reduction of wustite: FeO+[C] = Fe+CO. The direct reduction via carbon dissolution and diffusion in solid iron shell could thus compete with indirect reduction of wustite by carbon oxide restored via Boudouard reaction. With regard to this competition it is interesting to note that ability of carbonaceous matter to dissolve in iron (solid or liquid) depends on carbon crystallinity and is, roughly speaking, inversely proportional to its reactivity to carbon dioxide. Moreover, the carbon dissolution in iron is promoted by good contact between the particles of iron oxide and reducing agent, it means by relatively low mixture porosity (or interparticles void fraction). In contrary, Boudouard reaction kinetics needs rather high porosity (or void fraction) of self-reducing mixture to assure good interparticles diffusion conditions for gaseous reactants (CO and CO2).
Finally, it should be noted that the conditions of self-reduction at high temperatures can also be modified by the appearance of a liquid phase resulting from melting of ashes, gangue, mineral additives as well as reduced and carburized iron. Thus, taking all that into account, it is clear that controlling step changes when reduction progresses and this all the more so because in non-isothermal conditions the temperature of self-reducing mixture rises at the same time as fractional reduction progresses.
All discussed above the rate limiting factors fit well with the self-reduction in laboratory conditions where the relatively small samples, in form of crucible with purred powder or small (few millimetres in size) pellets or briquettes, are used. In such the samples and under a relatively low heating rate, a temperature gradient inside the reacting mixture could be neglected. However, for the pellets and briquettes of few centimetres in size, destined to industrial uses, the heat transfer could also interfere in the self-reducing process and become the rate controlling factor.21,29)
3.4. Morphology of Reduced IronIt is well known that the morphology of metallic iron produced from wustite depends on the reduction conditions, mainly the temperature and the reducing gas composition (specifically CO2/(CO+CO2) ratio).36,37) According to Gudenau et al.38) there are three types of iron morphologies: layered dense iron, porous iron and directed-whiskers.
In fact, in our earlier study3) on gaseous reduction of scale, two different morphologies of iron were observed: the porous iron, which prevailed in the samples reduced at higher temperatures (1100°C and 1200°C), and the iron whiskers formed in the scale samples reduced at lower temperatures (900°C and 1000°C). It was thus interesting to examine and compare the morphology of iron produced with different solid carbonaceous materials, in order to identify the conditions in which the self-reducing reactions occur.
Micrographs in Figs. 15(a) and 15(b) clearly show that self-reduction of scale with charcoal produces iron in form of whiskers, growing on the shrinking core of parent wustite particles. These iron whiskers have similar size and shape as those observed for gaseous reduction of scale at lower temperatures (900–1000°C). The morphology of iron attests that the self-reduction occurs at a relatively low temperature and it agrees with the kinetic measurements (Fig. 5), which show that during the heating the self-reduction of scale by charcoal starts at about 800°C and it is almost completed below 1100°C.

Partially reduced particles of scale: a) and b) reduced by charcoal (in the non-isothermal test with 20 K/min up to 1100°C) and exhibiting an iron shell made of whiskers; c) and d) reduced by petroleum coke (in the isothermal test at 1100°C) and exhibiting a shell of porous iron.
However, the micrographs presented in Figs. 15(c) and 16(d) show that self-reduction of scale with BF coke produces a porous iron, which is characteristic of higher temperature of reduction. In fact, the kinetic curve relating to BF coke used as a reductant (Fig. 5), shows that the reduction starts at temperature above 1000°C and around 90% of conversion occurs during the isothermal step at 1100°C. Consequently, the formation of porous iron is clearly governed by such high temperature reduction conditions.

Influence of reductants reactivity on the morphology of iron produced by self-reduction.
The results of the morphological study of iron produced by self-reduction could be compared with that reported by Nascimento et al.,28) who studied the microstructure of self-reducing pellets bearing iron ore and carbon. They also found that the typical morphology of reaction product in the step wustite to metallic iron was iron whiskers in the temperature range from 950 to 1050°C and porous iron at a temperature higher than 1150°C.
It is also worth to note that the micrographs of partially reduced scale, as that presented in Figs. 15(c) and 15(d), show that even such small particle of 50 μm in size exhibits an un-reacted core of dense wustite and porous shell of reduced iron.
Figure 16 summarises schematically the findings of kinetic study together with the results of morphological analysis in order to explain the growth of different iron forms during self-reduction based on the mechanisms of gaseous reduction proposed by Rist et al.36,37) and Gudenau et al.38) According to these authors, the formation of iron of different morphology is due to the relative rate of two broad mechanisms that operate simultaneously. The first of them is oxygen removal, involving transport in the gas phase outside the particle and reaction on the particle surface. The second is iron transport in wustite based on vacancy diffusion. The most important factors affecting the gaseous reduction mechanism are: gas composition which sets up its reducing potential and temperature which influences not only the rate of oxygen removal at the particle surface but also affects the iron transport inside wustite particle. Other influencing factors are the surface characteristics of wustite which impacts the iron nucleation density and rate as well as the solute cations which affect iron transport in wustite particles.
In gaseous reduction, a layered dense iron is formed at relatively low temperature (600–750°C) when wustite is exposed in atmosphere with high reduction potential which leads to high iron nucleation density. However at such temperature range the mobility of ions in wustite is relatively low and the iron nuclei can grow only laterally at the particle surface to form a dense iron layer enclosing the un-reacted wustite core. This form of iron was not observed in our study.
At intermediate temperature range (800–900°C) the lower reduction potential of gas, the lower nuclei density together with a relatively high diffusion rate of iron in wustite promote whiskers growth. Apparently such the conditions occur within self-reduction mixture when high reactive reductant, like charcoal is used, allowing sufficient gasification rate in the temperature range 800–1000°C. Finally, at high temperature range (1000–1200°C) a high reduction potential, a high nuclei density together with high iron diffusion rate in wustite favour growth of porous iron. In self-reduction experiences the low reactive reductants (BF coke and petcoke) shift both gasification and reduction reactions to this high temperature range thus creating conditions which enable formation of porous iron.
Once more it should be stressed that this study concerns the relationship between the reducing agents reactivity and formed iron morphology obtained with self-reducing mixtures in the temperature range 800–1100°C. At higher temperature (above 1100°C), the direct reduction and iron carburization change not only the reaction kinetics but also modify the reduced iron morphology. Iguchi and Endo23) observed the coalescence of reduced and carburized iron particles at temperature above 1150°C depending on the carburizing abilities of carbonaceous reducing agent. Finally, Flores et al.39) observed a partial or complete melting of self-reducing mixture of scale and petcoke in the temperature range of 1300–1500°C due to the reduced iron carburization.
Four carbonaceous materials were studied as the potential reductants for self-reducing briquettes allowing recycling mill scale in an EAF. Firstly, their kinetics of gasification in pure carbon dioxide was measured by thermogravimetry. It was found that the most reactive reductant is charcoal followed by coal char, BF coke and petroleum coke. The mean apparent activation energy of gasification of these reductants by CO2 was determined to be in the range of 230 to 270 kJ/mol.
The isothermal series of TG measurements show that the kinetics of self-reduction depends strongly on the reductant reactivity. The charcoal appears to be the best reductant with the lowest temperature of the beginning of reduction (about 800°C) and the highest rate of reaction. In contrast, it was found that the reduction by petroleum coke starts at higher temperature (above 1000°C) and advances slowly even at 1100°C, which was the maximal temperature in the reported experiments. Therefore, the charcoal was chosen for further study of industrial size self-reducing briquettes.
The non-isothermal TG measurements allowed for better insight into the kinetics and mechanism of the self-reduction of scale. It was found that at the beginning the self-reduction rate is controlled by the Boudouard reaction in all cases, i.e. when using low as well as high reactivity reductants. However, from the decrease of apparent activation energy with conversion it was concluded that the reduction influences (slows down) the overall rate of self-reduction at a higher reduction degree, especially when highly reactive reductant (charcoal) is used. Moreover, the self-reduction tests suggest that the tests of gasification by pure CO2 were not free of some mass transfer limitations. This is the reason for the higher apparent activation energies measured at the beginning of self-reduction comparatively to these obtained in the tests of gasification by pure CO2.
A microscopic analysis of the reduced sample has shown that different morphological forms of iron are produced when the reductant changes. Highly reactive charcoal, which is characterised by low temperature of self-reduction, promotes the iron whiskers formation, while low reactivity BF coke and petroleum coke, shifting the reduction to higher temperature, favour the formation of porous iron. However, in both cases, reduction of dense wustite particles proceeds in a topochemical way according to the un-reacted shrinking core model. The morphologies of iron produced could be thus used to identify the local conditions (temperature and gas composition) inside industrial size briquettes, in which direct measurements are not commonplace and easy to do.
The authors gratefully acknowledge the financial support from the Brazilian National Council of Scientific and Technological Development - CNPq/Program: Science Without Borders.