2014 Volume 54 Issue 12 Pages 2780-2789
2014 Volume 54 Issue 12 Pages 2780-2789
As a fundamental study to clarify the agglomeration and coalescence of alumina inclusions in molten steel from the viewpoint of interfacial chemical interactions, it has been experimentally verified for the first time that significant agglomeration force is exerted between alumina particles in aluminum deoxidized molten steel by using a newly established experimental method. In this method, the agglomeration force exerted between alumina particles in molten steel is directly measured separately from the effect of molten steel flow. In addition, it has been quantitatively demonstrated that the contact angles measured between aluminum deoxidized molten steel and an alumina plate are larger than those between the molten iron-oxygen alloy and the alumina plate, which have already been measured by other researchers. Moreover, it has also been indicated by analyzing the actual measurement values of agglomeration force with an interaction model taking contact angles and interfacial properties into consideration that the agglomeration force between the alumina particles in aluminum deoxidized molten steel derives not from the van der Waals force but from the cavity bridge force occurring due to molten steel, which is unlikely to wet the alumina particles. Meanwhile, it has been assumed that the agglomeration force on spherical alumina inclusions in aluminum deoxidized molten steel calculated on the basis of the interaction model according to the cavity bridge force is greater than the buoyant force and drag force, and the alumina inclusions once coming into contact are therefore not prone to be simply dissociated even under molten steel flow. Thus, they maintain the agglomeration state and are subsequently sintered and form comparatively solidly bonded alumina clusters.
Refined molten steel is usually deoxidized with relatively inexpensive aluminum having high deoxidizing ability. Generated alumina inclusions having approximately few microns in size form alumina clusters through collision, agglomeration, and coalescence, with some of them remaining in the continuously cast slabs, thus becoming the cause of surface and internal defects. In steelmaking processes, alumina inclusions, as coarse inclusions favorable for flotation, must be removed from the inside of molten steel by facilitating the agglomeration and coalescence of alumina inclusions by stirring or flow control of molten steel. In order to meet this need, it is extremely important to scientifically elucidate the agglomeration mechanism of alumina inclusions in molten steel.
In early studies on the inclusion behavior in molten steel, the kinetic elucidation was energetically performed on the deoxidation of molten steel based on the oxygen concentration variation in molten steel and particle size distribution of deoxidation products in rapidly quenched samples, which indicated that the deoxidation rates are controlled by the agglomeration and separation step of deoxidation products. Taniguchi et al. have recently given a detailed explanation of fluid dynamic actions such as Brownian agglomeration, agglomeration by laminar shear flow, turbulent agglomeration, and agglomeration by Stokes collision regarding the agglomeration and coalescence of inclusions.1) They conducted water model experiments2) and molten aluminum model experiments3) using quasi-inclusion particles and evaluated the behavior of turbulent agglomeration according to the agglomeration coefficient taking the van der Waals force into consideration as interparticle interaction. In addition, through in-situ observation on the behavior of inclusions drifting on the molten steel surface using a confocal scanning laser microscope combined with an infrared image furnace, Yin et al.4) indicated that powerful agglomeration force attributable to the capillary force is exerted especially between alumina inclusions. Moreover, Nakajima et al.5) indicated that the agglomeration force is intensively affected by the phase condition (solid phase, liquid phase, or solid-liquid phase) of an inclusion and its contact angle, by quantitatively analyzing the agglomeration force based on the capillary force as the interparticle interaction on the molten steel surface in consideration of size, shape, composition, and interfacial properties of inclusions. Thus, in addition to fluid dynamic actions, the importance of interfacial chemical interactions between particles has been pointed out regarding the agglomeration and coalescence of inclusions in molten steel. Nevertheless, the agglomeration mechanism of alumina inclusions in molten steel has not yet been adequately elucidated because it is difficult to independently extract only interfacial chemical interactions between inclusions in molten steel and to investigate them in detail based only on the size distribution evaluation of inclusions in a rapidly quenched sample, water model experiments and molten aluminum model experiments using quasi-inclusion particles, or a direct observation of inclusions on the molten steel surface.
In this paper, as a fundamental study to clarify the agglomeration mechanism of alumina inclusions in molten steel from the viewpoint of interfacial chemical interactions, the contact angle between aluminum deoxidized molten steel and an alumina plate was actually measured, its quantitative validity was verified, and the agglomeration force exerted between the alumina particles in molten steel was directly measured separately from the effect of molten steel flow. The origin of agglomeration force exerted between alumina particles in molten steel was identified by analyzing the actually measured agglomeration force using an interaction model, allowing for interfacial properties between molten steel and alumina particles. In addition, the mechanism where alumina inclusions maintain the agglomeration state under molten steel flow was also discussed by comparing the agglomeration force calculated from the interaction model with the buoyant force and drag force for the spherical alumina inclusions.
Steel samples for contact angle measurement were fabricated by cutting into cubes of 3 mm from solidified steel ingots, which were obtained from electrolytic iron (carbon concentration = 0.001 mass%, sulfur concentration = 0.0001 mass%, and oxygen concentration = 0.005 mass%) that was melted by high-frequency induction melting furnace under an Ar gas atmosphere, and deoxidized by adding a designated amount of Al at a molten steel temperature of 1600°C. The steel samples had an Al concentration in the range of 0.006–0.057 mass%. Al2O3 plates with a smoothly ground surface and a thickness of 7 mm were prepared by manufacturing the cylindrical tablets of 10 mm in diameter from an alumina reagent with a high purity of 99.9 mass% by one-axis compression formation under a pressure of 196 MPa and sintering for 90 min under an Ar gas atmosphere at a temperature of 1600°C.
A schematic view of the experimental apparatus used in the contact angle measurement is shown in Fig. 1. A resistance heating furnace with heating elements of graphite cylinders, heated by high-frequency induction coils, was used for the contact angle measurement experiment. An alumina tube of 12 mm inside diameter and 25 mm height with an Al2O3 plate arranged at the bottom was placed in an alumina crucible of 21 mm inside diameter and 45 mm height and then fixed with gaps at the bottom and the side filled with an alumina reagent. This complete alumina crucible was put into a graphite crucible of 52 mm inside diameter and 160 mm height, which was placed in the melting furnace. The steel sample of 3 mm cube with a designated Al concentration was loaded onto the Al2O3 plate and the temperature of the resistance heating furnace was raised to 1600°C under an Ar gas atmosphere. A drop of molten steel was brought into contact with the Al2O3 plate for 30 min before the power source of the melting furnace was disconnected, and this was solidified into the shape at the time of melting and then cooled to room temperature without any change. Photographs were taken of the front shape of the solidified specimens having a drop shape, which were taken out and placed on a flat table after the experiment. The average value of contact angles on the right and left, which were directly estimated from these photographs, was assumed to be the contact angle between molten steel and Al2O3 plate.

Schematic view of the experimental apparatus for contact angle measurement.
An experimental apparatus for measuring the agglomeration force between Al2O3 particles in molten steel is shown in Fig. 2. A resistance heating furnace with heating elements of graphite cylinders (graphite heater), heated by high-frequency induction coils, and a graphite crucible were used in the agglomeration force measurement experiment for the purpose of restraining the molten steel flow as much as possible. For the agglomeration force measurement, Al2O3 cylinders of a designated diameter were fastened vertically on the inner wall of an alumina crucible having an inner diameter of 40 mm and a height of 150 mm. On the other hand, Al2O3 cylinders with the same diameter and a length of 30 mm for the agglomeration force measurement were fastened to the lower end of an alumina protective tube of 8 mm in outer diameter and 380–440 mm in length. The upper end of this tube was connected to an aluminum rod on the melting furnace such that the Al2O3 cylinders could be arranged to be brought into contact with each other in parallel inside molten steel within the crucible. The alumina protective tube had such a structure that it could smoothly revolve around the rotation axle positioned 40 mm below the upper end of the aluminum rod. A force gauge was set on a moving stage on guide rails and linked to a driving motor via a wire. The force gauge was horizontally connected by a hook to a position 30 mm above the rotation axle of the aluminum rod, where when the aluminum rod was pulled by the driving motor, the traction force generated against the agglomeration force between the Al2O3 cylinders in molten steel was outputted on a chart recorder from the force gauge.

Schematic view of the experimental apparatus for agglomeration force measurement.
Six hundred grams of electrolytic iron having the same composition as that in the contact angle measurement experiment was melted in the alumina crucible with the Al2O3 cylinders fastened on the inner wall under an Ar gas atmosphere. After maintaining the temperature of molten steel at 1600°C, the molten steel was deoxidized by adding a specified amount of Al. The Al2O3 cylinders attached to the lower end of the alumina protective tube were immersed in molten steel and brought into contact with the Al2O3 cylinders on the inner wall of the crucible in parallel at a position 10 mm above the bottom of the crucible. As an experimental condition, the diameter of Al2O3 cylinders for the agglomeration force measurement was varied in a range of 6–10 mm. The moving stage was moved in the direction away from the aluminum rod at a velocity of 0.16 mm·s–1 by the driving motor, the traction force outputted from the force gauge was recorded on a chart recorder, and based on a change in the traction force, the agglomeration force was evaluated using a later-described method. All Al2O3 cylinders between which the agglomeration force was measured were made of Al2O3 with a high purity of 99.6 mass%. In order to grasp precisely the molten steel composition during the experiments, the molten steel was sampled using a transparent quartz tube of 6 mm in inner diameter before and after the agglomeration force measurement, and these samples were used for the analysis of Al concentration in molten steel. Al concentration in molten steel during the experiments ranged from 0.005 to 0.073 mass% as the average values of the analytical results before and after the agglomeration force measurement.
When a molten steel drop has a radius sufficiently small relative to the capillary length λCL (m) calculated from Eq. (1), the shape of the molten steel drop is dominated by surface tension rather than by gravity and can thus be considered to be a partial sphere.
| (1) |
| (2) |
As an example of the results of this contact angle measurement experiment, a photograph of a solidified drop specimen at an Al concentration [Al] of 0.057 mass%, which was shot from the front, is shown in Fig. 3. As expected from the calculation of capillary length, the solidified drop specimen represents a relatively uniform spherical shape. Accordingly, the comparison between directly measured contact angles and indirectly calculated contact angles by using Eq. (2) from the drop shape hD/dC read out from the photographs of solidified drop specimens is shown in Table 1. Both contact angles correspond approximately, and more precisely, the directly measured contact angles are slightly larger. Strictly, the drop shape becomes slightly flat due to gravity; thereby, the actual contact angles are marginally larger than the calculated values according to Eq. (2), where it is assumed that the drop shape is a sphere. Hence, it is judged that the directly measured contact angles are reasonable values, and high-precision evaluation can thus successfully be conducted.

Photograph of a solidified drop specimen obtained in the contact angle measurement experiment ([Al] = 0.057 mass%,
| [Al] | Measured
| Calculated
|
|---|---|---|
| 0.006 | 138.5 | 138.2 |
| 0.021 | 155.3 | 154.9 |
| 0.057 | 150.0 | 148.1 |
The effect of Al concentration in molten steel on the directly measured contact angle between molten steel and Al2O3 plate is shown in Fig. 4. Although the contact angle increases with Al concentration in molten steel, an approximately constant contact angle is exhibited when the Al concentration reaches 0.02 mass% or higher. This reason is presumed as follows. In the region where the Al concentration in the molten steel is less than 0.02 mass%, the oxygen concentration being an interfacial active element decreases as Al concentration increases, so that the contact angle increases. In contrast, in the region where the Al concentration is 0.02 mass% or more, the molten steel is sufficiently deoxidized and the effect of oxygen is negligible, so that the contact angle becomes constant. In this study, contact angles of 155.3° and 150.0° obtained in the sufficiently deoxidized molten steel at an Al concentration of 0.02 mass% or more were considered to be the contact angles between Al deoxidized molten steel and Al2O3 plate.

Effect of Al concentration in molten steel on the contact angle between molten steel and Al2O3 plate.
Regarding the two cases of Al concentration in molten steel, 0.005 mass% and 0.063 mass%, the changes in the traction force FT (N) outputted from the force gauge in the agglomeration force measurement experiment are shown in Fig. 5. Here dCY is the diameter of an Al2O3 cylinder (mm), LD is the distance from the rotation axle to the lower end of the Al2O3 cylinder (mm), LU is the distance from the rotation axle to the attachment position of the force gauge (mm), and L is the length of the Al2O3 cylinder (mm). In the experimental apparatus in Fig. 2, when the moving stage is moved to the side of the driving motor, force is applied in the direction separating the Al2O3 cylinders in molten steel away from each other, and thus the traction force measured by the force gauge gradually increases with time. Once the traction force separating the Al2O3 cylinders away from each other surpasses the agglomeration force between the Al2O3 cylinders, the two Al2O3 cylinders are instantly divided and the traction force sharply decreases. Even if the Al concentration in molten steel is varied, the behavior of the measured traction forces is the same and only the absolute value varies according to the molten steel compositions. Therefore, it is considered that the maximum traction force FT,Max (N) at the moment when the Al2O3 cylinders are separated from each other is equivalent to the agglomeration force between the Al2O3 cylinders in molten steel at the corresponding Al concentration. Accordingly, it can be seen that the agglomeration force between the Al2O3 cylinders in molten steel can be directly measured by using this experimental method according to the compositions of molten steel.

Changes in the traction force outputted from the force gauge in the agglomeration force measurement experiment.
The true agglomeration force FA (N·m–1) exerted between the Al2O3 cylinders is derived through applying the principle of leverage from the maximum traction force measured by the force gauge as shown in Eq. (3).
| (3) |

Effect of Al concentration in molten steel on the agglomeration force between Al2O3 cylinders in molten steel.

Relation between the agglomeration forces between Al2O3 cylinders in Al deoxidized molten steel and the diameter of Al2O3 cylinders.
Figure 8 shows a cross-sectional photograph of Al2O3 cylinders in a solidified steel ingot, which were brought into contact with each other in the molten steel at an Al concentration of 0.064 mass% and then rapidly cooled by turning off the power source of the melting furnace. It can be seen that cavity bridges are formed at the contact area between the Al2O3 cylinders in Al deoxidized molten steel.

Cross-sectional photograph of Al2O3 cylinders in a solidified steel ingot ([Al] = 0.064 mass%, dCY = 8 mm).
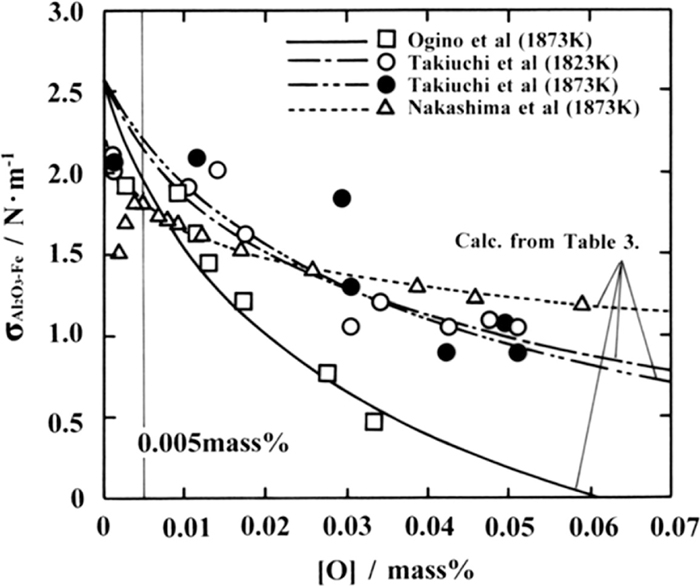
Figure 9 shows the effect of oxygen concentration [O] (mass%) in molten steel on the contact angle between molten steel and an Al2O3 plate. Furthermore, as previously mentioned, the oxygen concentration in molten steel in this experiment are equilibrium oxygen concentrations at 1600°C calculated from Al concentrations in molten steel by using the thermodynamic reevaluation value6) of the Al deoxidation equilibrium by Itoh et al., and those by Nakashima et al.,7) Takiuchi et al.,8,9) and Ogino et al.10) are oxygen analytical values of the Fe–O alloy that are not deoxidized with Al. According to their reports,7,8,9,10) the contact angle between molten steel and Al2O3 plate falls to approximately 115–135° as the oxygen concentration in molten steel decreases in the region where the oxygen concentration is not higher than 0.005 mass%. Nevertheless, a large contact angle in the range of 150.0–155.3° was obtained according to the measurement by the author. It has been reported7,8,9) that the decrease in the measured contact angle between the molten Fe–O alloy and Al2O3 plate in the region of low oxygen concentration results from the dissociation of Al2O3 plate. It is presumed that since the target of the measurement results by the author is the Al deoxidized molten steel with a dissolved Al of 0.02 mass% or more, there is no dissociation of the Al2O3 plate so that the contact angle becomes lager. However, this detail will be quantitatively discussed below.

Effect of oxygen concentration in molten steel on the contact angle between molten steel and Al2O3 plate.
The contact angle between molten steel and Al2O3 is expressed by the following Young’s equation:
| (4) |
Ogino et al.,10,11) Takiuchi et al.,8,9) and Nakashima et al.7) investigated the effect of oxygen concentration in molten steel on the surface tension of molten Fe–O alloy, and arranged those measurement results based on Szyszkowski’s equation12) capable of allowing for a reduction in the surface tension due to oxygen absorption as shown in Table 2. aO denotes the activity of oxygen in molten steel. Additionally, the experimental equation by Nakashima et al. in Table 2 was formulated by the author based on their measurement results7) according to the later-mentioned procedure as in the case of interfacial tension. Keene13) reported dσFe/d[Al]= –0.037 N·m–1·mass%–1 as the effect of Al concentration on the surface tension of molten Fe–Al alloy. Even if the Al concentration in molten steel increases to approximately 0.1 mass%, the reduction in the surface tension is no more than 0.0037 N·m–1, and is thus extremely small compared with a surface tension of 1.90–1.97 N·m–1 in molten pure iron. Accordingly, the effect is negligible in the Al concentration range of this study, and the experimental equation of Table 2 regarding molten Fe–O alloy thus allows the appropriate evaluation of the surface tension of molten Fe–Al–O alloy.
4.1.2. Formulation of Interfacial Tension between Molten Fe–Al–O Alloy and Al2O3 PlatesThe Szyszkowski’s equation12) of Eq. (5), in which oxygen in molten steel is considered to be an interfacial active element, is also applied to the interfacial tension between molten steel and Al2O3, as in the case of surface tension.
| (5) |
| (6) |
The effect of oxygen concentration in molten steel on the interfacial tension between molten steel and Al2O3 plate is shown in Fig. 10. The data in Fig. 10 are the interfacial tension between molten Fe–O alloy and Al2O3 sought from Eq. (4) by using the surface tensions in Table 2, contact angles in Fig. 9, and Al2O3 surface tension of 0.75 N·m–1. As in the case of contact angles in Fig. 9, when oxygen concentration in molten steel is lowered to 0.005 mass% or less, the reduction in interfacial tension due to the dissociation of Al2O3 occurs. However, no dissociation of Al2O3 occurs in molten Fe–Al–O alloy. Hence, no decrease but an increase in interfacial tension is expected along with Eq. (5) concomitantly with the reduction in oxygen concentration, even when the oxygen concentration decreases to 0.005 mass% or below. On the other hand, it was reported by Ogino et al.,14) Takiuchi et al.,8) and Nakashima et al.7) that FeO·Al2O3 (hercynite) is created on the interface between molten steel of high oxygen concentration and Al2O3 plates. According to McLean et al.,15) the oxygen concentration at which Al2O3 and FeO·Al2O3 coexist is 0058 mass% at 1600°C; therefore, specifically, the data at this oxygen concentration or higher could be affected by FeO·Al2O3 existing on the interface. Accordingly, the interfacial tension between molten Fe–Al–O alloy and Al2O3 can be formulated by Eq. (5) satisfying the data in Fig. 10 at an oxygen concentration of 0.005–0.058 mass% in molten steel without Al2O3 dissociation or FeO–Al2O3 formation. Respective
Effect of oxygen concentration in molten steel on the interfacial tension between molten steel and Al2O3 plate.
|
|
|
|
|
|
|
|
The contact angle between molten Fe–Al–O alloy and Al2O3 calculated by substituting the surface tensions in Table 2 and interfacial tension in Table 3, formulated with respect to molten Fe–Al–O alloy for Eq. (4), is shown in Fig. 9 for respective researchers. Considering the variation in actual measurement values, the calculated contact angle comparatively agrees with the corresponding actual measurement value by each researcher under the condition without either Al2O3 dissociation or FeO–Al2O3 formation on the interface, i.e., at an oxygen concentration range of 0.005–0.058 mass%. Consequently, it is considered that the calculated contact angle also correctly reflects the contact angle between molten Fe–Al–O alloy and Al2O3 in the region of low oxygen concentration of 0.005 mass% or below.
Accordingly, the validity of the measured contact angles between Al deoxidized molten steel and Al2O3 in this experiment will be verified below by comparing them with the calculated results. Since both the contact angle in Fig. 4 and agglomeration force in Fig. 6 indicate a steady value at an Al concentration of 0.02 mass% or higher, an equilibrium oxygen concentration of 0.0009 mass% is obtained6) by assuming the representative Al concentration of Al deoxidized molten steel to be 0.02 mass%. As can be seen from Fig. 9, the estimated contact angles between Al deoxidized molten steel and Al2O3 at this oxygen concentration are 156.4° (by Ogino et al. at 1600°C), 154.8° and 160.2° (by Takiuchi et al. at 1550°C and 1600°C, respectively), and 134.0° (by Nakashima et al. at 1600°C), with an average of 151.3°. The actually measured contact angles between Al deoxidized molten steel and Al2O3, 150.0° and 155.3°, lie within the range of contact angles calculated by each researcher (a range of 134.0–160.2° between the dotted line and two-dot chain line), and the average of these contact angles, 152.6°, indicates a good correspondence with the average value of previously calculated contact angles. Therefore, it is judged that the average contact angle of 152.6° sought from the actually measured values is an appropriate value as the contact angle between Al deoxidized molten steel and Al2O3.
4.2. Origin of Agglomeration Force Exerted between Al2O3 Cylinders in Molten SteelThe agglomeration forces exerted between the Al2O3 cylinders in molten steel are considered to include: (a) liquid bridge force due to molten oxide that is prone to wet Al2O3, (b) van der Waals force, and (c) cavity bridge force attributed to the fact that molten steel is unlikely to wet Al2O3.17,18,19)
Regarding (a), the authors have reported that molten FeO produced by significant reoxidation of molten steel or molten TiO2–CaO–Al2O3 produced with Ca treatment forms a liquid bridge between Al2O3 inclusions, which agglomerate with each other in molten steel.17,18,19) However, because of the fact that molten steel was sufficiently deoxidized with Al in the present experiments as in ordinary Al deoxidized molten steel so that no molten oxides such as FeO existed in molten steel and that no molten oxides were observed between Al2O3 cylinders, it is inconceivable that the agglomeration force between Al2O3 cylinders is caused by the liquid bridge force (a).
Consequently, whether the agglomeration force exerted between the Al2O3 cylinders in molten steel arises from the van der Waals force (b) or the cavity bridge force (c) will be quantitatively analyzed below by using the interaction model for each.
4.2.1. Agglomeration Force Due to van der Waals ForceIn general, an interaction that is exerted when solid particles mutually approach in liquid principally includes the repulsion force owing to the overlapping of diffuse electric double layers and the dispersion force (van der Waals force). Since it is not necessary to consider the diffuse electric double layer in the Al2O3 particles in molten steel,1) the agglomeration force alone due to van der Waals force, FA,V (N·m–1), approximately represented by Eq. (7) acts on the two isodiametric Al2O3 cylinders in the present experiments.20)
| (7) |
| (8) |
When the Al2O3 cylinders having poor wettability with molten steel come into contact with each other, a cavity bridge is formed between them, as shown in Fig. 8. In this case, the agglomeration force FA,S (N·m–1) created between two isodiametric Al2O3 cylinders is expressed as the sum of the pressure difference ∆PFe (Pa) between cavity bridge and molten steel, and the surface tension of molten steel, which is given by Eq. (9).
| (9) |
| (10) |
| (11) |
| (12) |
| (13) |
An average value of 1.884 N·m–1 on the calculated surface tensions at the equilibrium oxygen concentration of Al deoxidized molten steel of 0.0009 mass%, according to the respective equations in Table 2, was considered to be the surface tension of Al deoxidized molten steel. In addition, the contact angle between Al deoxidized molten steel and Al2O3 was 152.6°. By using these values and the expected values of ∆PFe, the agglomeration force per unit length between two isodiametric Al2O3 cylinders, calculated according to Eqs. (9) and (13), is shown in Fig. 7. Although not precisely known, the pressure in the cavity bridge may be considered to be 8.2 Pa corresponding to the vapor pressure of Fe (at 1600°C) or higher, and simultaneously, lower than the addition of the static pressure of molten steel and atmospheric pressure 1.05×105 Pa. When the pressure within the cavity bridge is equal to the vapor pressure of Fe, i.e., when it is assumed that ∆PFe is 1.05×105 Pa, the calculation value of the agglomeration force, indicated by the dotted line in Fig. 7, results in being approximately five times higher than the corresponding experimental value. However, both values are broadly equivalent with respect to the order of magnitude, and the diameter dependency of Al2O3 cylinders on the agglomeration force is analogous in both. Moreover, assuming that the pressure within the cavity bridge is 1.01×105 Pa, ∆PFe becomes 3.86×103 Pa, and the calculated agglomeration force indicated by the solid line thus successfully agrees with the experimental value. Furthermore, when ∆PFe is 3.86×103 Pa, X4 obtained from Eq. (13) is found to be 1.44 mm, which approximately corresponds to 1.35 mm of the half-width of the neck of the cavity bridge in Fig. 8.
From the above mentioned results, the agglomeration force exerted between Al2O3 particles in molten steel is presumed to originate in the cavity bridge force between Al2O3 particles, which is unlikely to be wet with Al deoxidized molten steel.
4.3. Mechanism for Agglomeration Maintenance of Al2O3 Inclusions in Molten SteelBased on these experimental results, the agglomeration force exerted on spherical Al2O3 inclusions in Al deoxidized molten steel is estimated below. As shown in Fig. 8, when a cavity bridge between two isospherical Al2O3 inclusions is formed, the agglomeration force FA,S (N) is expressed by Eq. (14).
| (14) |
| (15) |
| (16) |
| (17) |
| (18) |
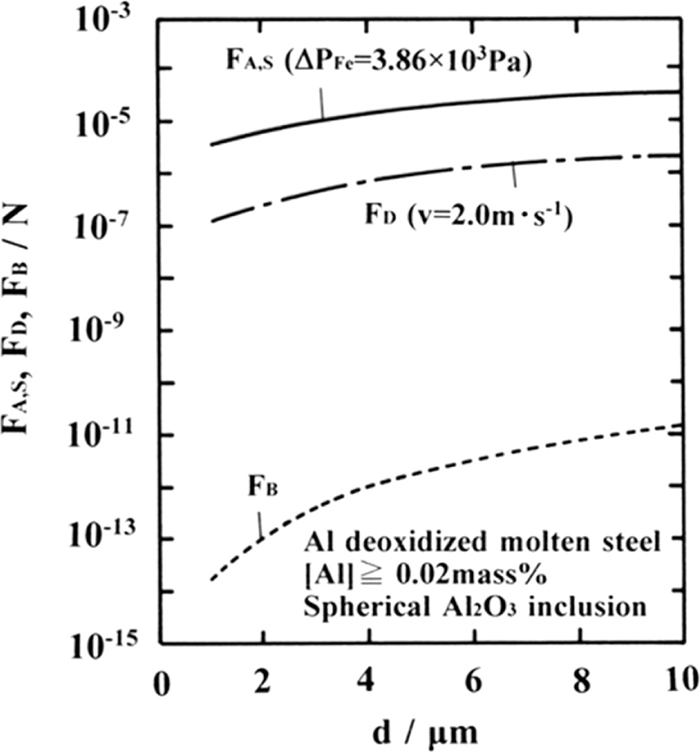
Relation between the agglomeration force between two isospherical Al2O3 inclusions due to cavity bridge force and the diameter of Al2O3 inclusions.
The buoyant force FB (N) exerted on Al2O3 inclusions in molten steel can be evaluated using Eq. (19).
| (19) |
| (20) |
| (21) |
As a fundamental study for revealing the agglomeration and coalescence of Al2O3 inclusions in molten steel in view of the interfacial chemical interaction, direct measurement of the agglomeration force exerted between Al2O3 particles in molten steel was performed separately from the effects of molten steel flow, and the contact angle between Al deoxidized molten steel and Al2O3 plates was actually measured, where it was quantitatively verified that these values are valid. In addition, based on these results and an interaction model considering interfacial properties, the origin of the agglomeration force exerted between Al2O3 particles in molten steel and mechanism by which Al2O3 inclusions maintain their agglomeration state under molten steel flow were analyzed. The conclusions obtained are as follows:
(1) The contact angle between Al deoxidized molten steel (Al concentration≧0.02 mass%) and Al2O3 plates is 152.6°, and indicates a larger value than those between the molten Fe–O alloy and Al2O3 plates already measured by other researchers. This is explained by the fact that the dissociation of Al2O3 plates occurs in the molten Fe–O alloy, whereas it does not occur in Al deoxidized molten steel under the influence of dissolved Al.
(2) The surface tension of the molten Fe–Al–O alloy as well as its interfacial tension of molten Fe–Al–O alloy with Al2O3 plates was formulated according to the surface tension of molten Fe–O alloy and the contact angle of molten Fe–O alloy with Al2O3 plates measured by Nakashima et al., Takiuchi et al., and Ogino et al., and the contact angle between molten Fe–Al–O alloy and Al2O3 plates was quantitatively estimated by applying these formulated functions to Young’s equation. The values were validated by comparing the contact angles between Al deoxidized molten steel and Al2O3 plates obtained in this study with these estimated values.
(3) A new experimental method for direct measurement of the agglomeration force exerted between the Al2O3 particles in molten steel separately from the effect of molten steel flow was established, and by using this method, it was practically verified for the first time that a large agglomeration force is exerted between Al2O3 particles in Al deoxidized molten steel.
(4) The agglomeration force between Al2O3 particles in Al deoxidized molten steel is caused not by the van der Waals force but by the cavity bridge force created due to Al2O3 particles, which is unlikely to be wet with molten steel.
(5) The agglomeration force between Al2O3 inclusions due to the cavity bridge force in Al deoxidized molten steel is large compared with the buoyant force and drag force exerted on Al2O3 inclusions; therefore, once the Al2O3 inclusions come into contact with each other, they maintain their agglomeration state without being easily separated even under a flow of molten steel and are subsequently sintered and form relatively firmly bonded alumina clusters.