2014 Volume 54 Issue 3 Pages 613-619
2014 Volume 54 Issue 3 Pages 613-619
In-depth investigations were carried out on the thermal degradation and structural evolution of bakelite by heat treatment at different temperatures; the structural transformation to graphitic carbon at 1450°C was confirmed through X-ray diffraction. High amounts of residual carbon were obtained after the high temperature charring of bakelite. The reduction behavior of iron oxide/bakelite composite pellets was studied at 1450°C to investigate waste bakelite as a carbon resource in ironmaking towards a partial replacement of traditional carbon sources. These studies were carried out for raw bakelite as well as for bakelite char. The reduction of iron oxide by raw bakelite resulted in the non-separation of metal, slag and in the formation of direct reduced iron pellets. On the other hand, bakelite char pellet showed clear separation of iron nuggets from slag. This study has established bakelite as an alternative carbonaceous resource for reduction reactions in new ironmaking processes.
The iron and steel industry is one of the largest industrial sources of CO2 emissions due to its reliance on carbon-based fuels and reductants. With over 1518 Mt of steel produced in 2011,1) steelmakers are faced with finding ways of lowering CO2 emissions and reducing their carbon footprint without seriously affecting the process efficiency.2) The average energy consumption in the steel industry is about 10% of the national gross energy consumption in countries such as China. The cost of energy in the cost of steel production is ~30%. In addition to the cost factor, nearly 70% of energy is produced from coal-based thermal power stations, which also produce huge amounts of CO2 gas.3) Energy has therefore become one of the most important factors in the steel industry, impacting the cost and environment greatly. Blast furnace is still the primary method for ironmaking which consumes enormous amounts of energy and generates the most CO2 emissions. The main process consuming the highest amounts of energy is the reduction of iron ore and the carburization of reduced iron at high temperatures. In recent years, there has been considerable effort towards developing alternative ironmaking routes; the use of carbon composite pellets is one such advantageous technology that uses briquetted mixtures of iron ore/fines with a range of carbonaceous materials such as pulverized coal, coke breeze, char, anthracite, charcoal, petroleum coke etc. to decrease the consumption of raw materials fuel, reduce emissions and enhance waste utilization.
Halder et al.4,5) explored the use of coal char and devolatilized wood charcoal in composite pellets based on the concept of a rotary hearth furnace (RHF) of utilizing direct reduced iron (DRI) pellets. The internal heat transport was found to be a rate-limiting step during the top-layer reduction of pellets. Wood charcoal appeared to be a faster reductant than coal char. Pellets also showed shrinkage due to the loss of carbon, sintering of hematite and the formation of molten slag; the shrinkage was found to be temperature dependent. Anameric et al.6) studied process requirements including the dependence on residence time for the production of iron nuggets at 1425°C based on a single step pig iron making process (Kobe Steel’s ITmk3®). Significant levels of carbon dissolution and the formation of pig iron nuggets was achieved after a residence time of 40 minutes.
Several investigations have been carried out on CO2-neutral/waste carbonaceous materials such as waste plastics, biomass, devolatilised wood charcoal etc. The global consumption of plastics is fast approaching 100 million tonnes per year, of which only a small fraction is currently recycled. In Australia, only ~20% of plastic products were recycled in 2009-10 with the majority being thermoplastics such as polyethylene (PE) and polyethylene terephthalate (PET); only a small fraction of thermoset plastics (0.4%) were recycled among these plastics.7) As waste plastics contain high percentages of both carbon and hydrogen; these could potentially be used for the reduction of iron oxide and could play an important role as a fuel/carbon resource for iron and steelmaking.8,9,10,11,12,13,14,15,16)
Matsuda et al.17,18) have reported on the reduction behaviour of iron oxide by waste PE and wood. Mixtures of waste PE/wood and hematite were blended in a range of C/O molar ratios; these were heated rapidly to temperatures between 1400°C and 1800°C. The metal yield showed a maximum at C/O ratio≈1 for PE-hematite mixture whereas the maximum C/O ratio for wood mixtures was found to be 0.75. Ueki et al.19) investigated reactions between waste plastics (PE and refuse derived fuel) and iron oxide blends at high temperatures (1000–1300°C) in Ar gas. The thermal decomposition of waste plastics at 1300°C resulted in the generation of significant amounts of reducing gases: H2 (~60–70%) and CO (~70–80%), both of which took part in reduction reactions. The reduction reaction with polyethylene however did not reach completion and the reaction was found to stagnate. This was attributed to the rapid release of gases and loss of carbon as volatiles and the generation of small yields of solid residual carbon. On the other hand, the reduction degree achieved with refuse derived fuel was much higher due to significant amounts of char generated during thermal decomposition.
One of the main limitations of using thermoplastics in composite pellets is the low yield of pyrolysis residue and high rates of volatile release at ironmaking temperatures.20,21) The residual carbon yield of a number of thermoplastics has been reported during pyrolysis at 740°C as: Polyvinylchloride (PVC) - 9%; Polyethylene (PE) - 1.8%; Polypropylene (PP) - 1.6%; Polystyrene (PS) - 0.6%. Chars produced during pyrolysis at 850°C in N2 atmosphere from PVC, PE and PP were determined to be 5.9%, 0.2% and 2.3% respectively. On the other hand, thermoset plastics are expected to produce significant quantities of residual carbon and relatively lower levels of volatile release on exposure to high temperatures due to their complex bonding network and have the potential to be a suitable carbon resource for iron ore composite pellets.
In this article, we report in-depth investigations on thermal degradation and structural evolution of bakelite and high temperature reduction reactions of iron oxide-bakelite blends in composite pellets. Bakelite is a 3-dimensional cross-linked network structured thermoset polymer with high hardness, rigidity along with good thermal and electrical insulating properties. Commercial waste bakelite22) also contains significant levels (up to 30%) of calcium carbonate (CaCO3) as filler. Iron-oxide composite pellets were prepared with raw bakelite as well as with bakelite char in an attempt to develop guidelines for efficient, cost-effective waste recycling and productivity.
The ultimate analysis of bakelite, as determined by LECO analysis, was: 53.24% carbon, 4.02% hydrogen, 11.6% oxygen, 0.13% sulphur. The proximate and ash analysis of bakelite is shown in Table 1. Bakelite was ground to an upper size limit of 125 μm using a ring mill. The iron oxide used for production of the pellets was hematite (99% pure) with a particle diameter of 5 μm. Commercial bakelite contains CaCO3 (up to 30%) as filler which could be effectively used as a fluxing agent. This eliminates the need of additional fluxing agents in composite pellets.
| (a) | |
| Composition (%) | |
| Fixed Carbon | 31.70 |
| Ash | 17.77 |
| Volatiles | 47.55 |
| Moisture | 3.01 |
| (b) | |
| Compound | Wt % |
| CaO | 94.91 |
| SiO2 | 5.14 |
| SO3 | 0.33 |
Bentonite was used as a binder; it contained SiO2: 56.21, Al2O3: 17.63, Fe2O3: 3.15 and MgO: 2.18 as oxides components. The mixture used for making pellets was: 68.58% hematite, 24.9% carbonaceous material (raw bakelite as well as its char) and 6.51% binder. All materials were weighed using an analytical balance. The charring of bakelite was carried out at a range of temperatures in electrically heated horizontal tube furnace in argon atmosphere at a flow rate of 1.0 L/min. A schematic diagram of the horizontal tube furnace is shown in Fig. 1.

Schematic diagram of horizontal tube furnace.
Pellets were prepared using raw bakelite and bakelite char. Bakelite was charred at 1450°C for 20 min. The chemical composition of bakelite char was: 73.29% carbon, 9.12% oxygen, 0.06% sulphur. It contained 26.62% ash content. Pellets were made in a spherical shape using a cold bonding method by adding some water to the powder mixture of hematite, carbonaceous material and binder, which allowed the formation of a paste and homogenous blend. The diameter of each individual pellet was approximately 15 mm; these were placed in a drier at 100°C for further hardening.
2.2. High Temperature ReactionsThe structural transformations in bakelite and char formation were investigated at temperatures ranging between 350–1450°C; the heat treatment was carried out for 20 minutes at these temperatures. The residue collected was analyzed using X-ray diffraction and FTIR spectroscopy. A thermo gravimetric analyzer (TGA) was employed to study the weight loss as a function of temperature; a 10 mg bakelite sample was examined at a heating rate 10°C/min in N2 atmosphere. The reduction behavior of composite pellets was investigated in electrically heated laboratory-scale horizontal tube furnace. These studies were carried out at 1450°C in argon atmosphere at a flow rate of 1.0 L/min for different furnace residence time (10 min and 20 min). These experimental conditions (temperature/time) were expected to lead to the thermal decomposition of bakelite, reduction of iron oxide and subsequent carburization of reduced iron. A pellet was placed in an alumina crucible which was then placed on a graphite tray connected to a graphite rod. The graphite tray was kept in the cold zone (~300°C) for five minutes to avoid thermal shock, and then the reaction assembly was pushed into the hot zone and kept in this zone for 10–20 minutes. The pellet was then pulled back into the cold zone and kept there for few minutes to minimize the exposure of hot pellet to air and its subsequent oxidation.
Off gas analysis was carried out using an IR analyzer to record the amounts of CO and CO2 released as a function of time. These concentrations were then used to determine the reaction kinetics and the cumulative volumes of gases generated during reactions. The structural characterization of reduced pellets was carried out using X-ray diffraction. X-ray diffraction system was used with Copper Kα source operated at 45 kV and 40 mA. Samples were scanned with a step size of 0.026° and a scan rate of 1°/min. Phase identification was carried out using X’pert High score software. Scanning electron microscopic (SEM) investigations were carried out to investigate the topographical and compositional properties of pellets. Reduced pellets were analyzed using an SEM at accelerating voltages of 15–20 kV and a probe current of 50–70 mA.
The weight loss measurements of bakelite were carried out in a Thermo Gravimetric Analyzer (TGA) to temperatures up to 1300°C in N2 atmosphere. The TGA graph (Fig. 2) shows the weight loss due to thermal decomposition of bakelite and calcium carbonate. The TGA plot showed significant levels of residual char at 800°C; not much change was observed at higher temperatures. The DTG curve primarily showed two maxima’s (350°C and 452°C) that correspond to the thermal degradation of bakelite; weight losses in the temperature range 600–800°C are attributed to the degradation of both bakelite and calcium carbonate.
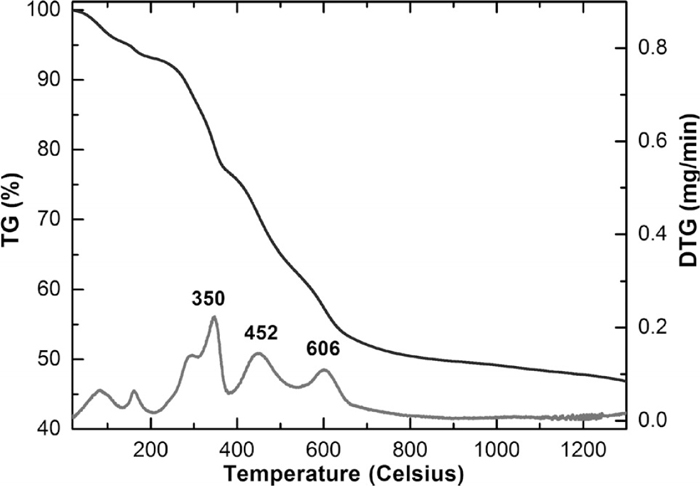
TG/DTG curve of bakelite degradation.
During the pyrolysis of bakelite, the volatile matter gets released leaving behind a solid residue with certain physical and chemical structure, which was characterized using IR spectroscopy. Figure 3 shows FTIR curves for raw bakelite and its residue after thermal degradation at 350°C. At 350°C, the OH stretching band at 3337 cm–1 was seen to shift towards higher wavenumbers due to reduced levels of OH groups.23) The thermal degradation mechanism of bakelite involves the fragmentation and dehydration of polymeric chains. The majority of bands were found to be decreased in intensity at 350°C. After heating at 450°C, the intensity of OH bands decreased further. Calcium carbonate showed peaks at 1440 cm–1 and 863 cm–1 in the IR spectrum. These peaks correspond to the vibration of carbon-oxygen double bond in the carbonate ion. The thermal degradation of CaCO3 starts at 735°C.

FTIR curve of raw bakelite and bakelite heated at 350°C.
The structural evolution of bakelite was investigated in the temperature range 350–1450°C and was analyzed by X-ray diffraction. Figure 4(a) shows XRD patterns of raw bakelite and its char at 1450°C. XRD diffraction patterns from chars in the temperature range 350–1300°C are shown in Fig. 4(b).

(a): XRD pattern of raw bakelite and bakelite char at 1450°C. (b): XRD pattern of bakelite heated at different temperatures.
Raw bakelite shows peaks due to amorphous carbon and a number of peaks due to CaCO3 and a small peak for SiO2. An almost similar X-ray diffraction pattern is observed after heat treatment at 350°C. The first evidence for thermal decomposition of CaCO3 was observed at 750°C in the form of small peaks for CaO. The decomposition of CaCO3 was seen to reach completion at 1150°C; diffraction peaks for Ca2SiO4 were observed in addition to CaO peaks. A graphitic carbon peak also made its appearance at this temperature. The heat treatment at 1300°C produced a much stronger carbon peak and additional peaks for Ca2SiO4, CaO and CaC2. The diffraction pattern at 1450°C showed most of these impurity peaks; the carbon peak however showed a significant increase in intensity. The crystallite height (Lc) of carbon was calculated by Scherrer formula:
| (1) |
A representative example of an un-reduced and three reduced composite pellets is shown in Fig. 5. Even though it had a large number of cracks on the surface, the pellet containing raw bakelite maintained its physical integrity after reduction reactions at 20 minutes (Fig. 5(b)). The size of the reduced pellet was however found to be considerably smaller than the unreduced pellet. On the other hand, bakelite-char pellets showed clear separation of metal and slag phases along with small amounts of residual bakelite char for 10 and 20 minutes of residence time (Figs. 5(c) and 5(d)).

Macro views of: a) and b) un-reduced and reduced bakelite based pellets respectively; c) and d) reduced bakelite char based pellets at 10 min and 20 min residence time respectively.
Figure 6 shows XRD patterns from raw hematite and reduced composite pellets. In reduced raw bakelite based pellet, a prominent peak of Fe for crystal plane (110) was observed along with a number of small intensity wustite peaks which tended to decrease in intensity with increasing time. Bakelite char based pellets on the other hand showed only completely reduced metal and no peaks for iron oxide. The degree of metallization of reduced raw bakelite based pellets was determined from the area under the curves of various peaks, whereas in bakelite-char pellet degree of metallization was computed using the weight of the metal products.

XRD of reduced pellets at 1450°C.
Raw bakelite based pellet showed 83.9% reduction of iron oxide, whereas bakelite-char pellet showed 90.12% metallization after 20 minutes of reaction. The metallization degree was also investigated as a function of reaction time (10 and 20 min) and was found to increase with increasing reaction time (Table 2). The carbon content in reduced iron determined using a LECO analyzer is also shown in Table 2. The carbon content of reduced metal in bakelite char pellet was found to be much higher (2.01 wt%) as compared to the raw bakelite pellet (0.23%) after 20 minutes of reaction. High levels of carbon in reduced iron for bakelite char pellets led to the local melting of iron and the formation of solid iron nuggets upon cooling; it also resulted in the separation of metal and slag.25) On the other hand, metal-slag separation did not occur in raw bakelite pellets probably due to relatively small amounts of carbon dissolved in iron and non-melting of iron.
| Carbonaceous Material | Metallization Degree | Carbon pick-up by iron | ||
|---|---|---|---|---|
| 10 min | 20 min | 10 min | 20 min | |
| Raw bakelite | 80.64 | 83.90 | 0.20 | 0.23 |
| Bakelite char | 85.12 | 90.12 | 1.89 | 2.01 |
The reaction kinetics and off-gases released from composite pellets was measured by IR-gas analyzer. Figure 7(a) shows plots of off-gases generated in the hot zone as a function of time; the cumulative moles for CO and CO2 generated were 1.39×10–2 and 1.54×10–3 respectively by raw bakelite and 2.04×10–2 and 0.94×10–3 respectively by bakelite char after 20 minutes of reaction. The CO gas was released over a longer time period in bakelite char based pellet as compared to the raw bakelite pellet. The inset views in Fig. 7(a) show ln(PCO/PCO2) plots against time for raw bakelite and bakelite char based pellet. At 1450°C, the equilibrium value of ln(PCO/PCO2) is about 9.02 and 1.01 for the Boudouard reaction and reduction of FeO by CO, respectively. The reduced fraction as a function of time for raw bakelite and bakelite char based pellet is given in Fig. 7(b). The overall reaction can be divided into three distinct stages as indicated by changes in slope. Regions 1 and 3 respectively indicate the initial contact and final equilibrium stages. Figure 7(c) shows linear region indicating stage 2 in reduced fraction (F) vs time plot for raw bakelite based pellet. In the case of bakelite char based pellet, stage 2 indicated the presence of a curvature and was divided into two separate steps (Fig. 7(d)). In stage 2a, reduced fraction (F) showed linear variation with time, while stage 2 b showed a good fit with –ln(1-F) variation with time.

(a): Off-gas concentrations (Vol%) and ln(PCO/PCO2) plots as a function of time during the reduction of iron oxide by raw bakelite (left) and bakelite char (right). (b): Degree of reduction of iron oxide as a function of time by raw bakelite and bakelite char. (c): Different stages during the reduction of iron oxide by raw bakelite. (d): Different stages during the reduction of iron oxide with bakelite char.
The oxygen contents of un-reduced and reduced composite pellets were determined using a LECO analyzer. For raw bakelite pellets, these were found to be 28.53% and 11.54% before and after reduction reactions. The corresponding numbers for the unreduced char based pellet and reduced metal droplet were 24.68% and 0.095% respectively.
Identification of various phases present in the reduced pellets was carried out using SEM. Figure 8 shows backscattered electron image for reduced reduce raw bakelite containing pellet at 20 minutes of reaction. This image clearly indicates presence of reduced iron along with wustite and slag phase (confirmed by EDX analysis). This image clearly reveals the presence of wustite dendrites in the slag matrix. The composition of slag matrix was determined to be a CaO–SiO2–Al2O3 oxide system along with small traces of other oxides. The slag phases also showed the presence of some pores due to various slag-forming reactions and release of gases.

SEM/EDS analysis of cross section of reduced raw bakelite based pellet at various magnifications after 20 minutes of reaction.
There were two key features of structural evolution of bakelite at high temperatures. As expected, there was a high yield of residual carbonaceous material with high carbon content (73%C) after exposure to high temperatures. The second key feature was the presence of a strong graphitic carbon peak, which made its first appearance after heat treatment at 1150°C but grew strongly in intensity with increasing temperatures. The relative intensity of the carbon (002) peak for raw bakelite was ~2000 units whereas the peak height for the C(002) peak in bakelite char after heat treatment at 1450°C showed a six fold increase (Fig. 4). It is believed that the origin of this graphitic carbon lies in the chemical reaction between CaO (from the thermal degradation of filler impurity CaCO3) and amorphous residual carbon which forms CaC2 and then dissociates to produce graphitic carbon.26,27) These results show that the carbon present in bakelite char was significantly more ordered than in raw bakelite; a characteristic that is highly desirable for enhancing carburization of iron.
In the composite pellet, the close contact between the reacting materials and the availability of large number of reacting sites enhanced direct reduction reactions; in addition, indirect reduction also took place with CO and CH4 gases. It is well known that the reduction of iron oxide takes place in a sequential manner: Fe2O3→Fe3O4→FeO→Fe.28,29,30) The reduction in composites is generally controlled by boudouard reaction (CO2 + C = 2CO) and wustite reduction by CO. The reduction of FeO to Fe is generally much slower than the reduction of Fe2O3 and Fe3O4. For pellets using raw bakelite, there were no peaks for Fe2O3 and Fe3O4 in the XRD spectrum thereby indicating the completion of these two steps of iron oxide reduction. Only peaks observed were for FeO and Fe pointing to an incomplete reduction of FeO. On the other hand, bakelite char containing pellets showed complete reduction to Fe. Otsuka and Kunii29) have found that the reduction of FeO to Fe was significantly enhanced when graphite was used as a reductant; this increase was attributed to the catalytic activity of metallic iron on the gasification of carbon. The presence of graphitic carbon in bakelite char could therefore have played an important role in enhancing the reduction of wustite to iron. In addition, CaC2 present as impurity in bakelite char is also known to reduce FeO to Fe (FeO+CaC2 = CaO + CO + Fe).31) The generation of CO gas for bakelite char was found to be sporadic and discontinuous indicating the occurrence of a number of reactions taking place over a period of time.
Raw bakelite based pellet showed a linear variation in reduced fraction (F) vs time plot in stage 2. In bakelite char based pellet, stage 2 was divided in two steps: in stage 2a, reduced fraction (F) showed a linear variation with time, while stage 2b showed –ln(1-F) variation with time. A linear fit to –ln(1-F) indicates a uniform internal reaction kinetics. In bakelite char based pellet, the generation of CO gas was discontinuous as compared to raw bakelite based pellet (Fig. 7(a)) which caused a non-linearity in stage 2 in reduced fraction (F) vs time plot in bakelite char based pellet and a change of slope (Fig. 7(d)). An examination of PCO/PCO2 provides additional insights into possible rate controlling mechanisms,32) where the PCO/PCO2 ratio is indicative of the oxygen partial pressure and can be related to the equilibrium oxygen partial pressures. In bakelite char based pellet ln(PCO/PCO2) value (see insets in Fig. 7(a)) showed deviation away from the FeO → Fe equilibrium towards the Boudouard reaction. However after 4 minutes of reaction, these values started to deviate away from the Boudouard equilibrium. A similar trend was observed for raw bakelite based pellet as well. The reaction rate appeared to be controlled by the carbon oxidation by CO2 and wustite reduction by CO in both composite pellets.
The key characteristics of raw bakelite pellet were the presence of amorphous carbon, up to 30% of CaCO3 impurities that could act as fluxing agents, in-situ generation of CO2 gas from thermal degradation of CaCO3, small levels of silica, and release of CH4 that could also take part in reduction reactions. The key features of bakelite char pellets were the presence of graphitic carbon, and slag impurities such as Ca2SiO4, SiC, CaO and CaC2. These distinct features led to significant differences in their iron oxide reduction behaviour. During initial stages, the rate of CO generation was higher for raw bakelite pellet along with the generation of small levels of CH4. Both these aspects resulted in higher rates of iron oxide reduction for raw bakelite pellet initially. Another key difference between raw bakelite and bakelite char was in their pore structure and distribution. Figure 9 shows high magnification SEM images (20 μm) of raw bakelite and bakelite char. Raw bakelite had a very small number of nano-pores whereas bakelite char showed significantly higher number of micro-pores. These pores can play an important role in the passage of gases through the solid phases, the availability of surface area of reaction and could significantly affect the kinetics of chemical reactions. Boudouard reaction is also strongly influenced by the surface area of carbon composite pellet because of more exposure to CO and CO2 gas stream.

SEM images of raw bakelite and bakelite char at 1450°C.
The reduction behavior of iron oxide pellets was investigated using raw bakelite and bakelite char as a reductant. Our results have shown that thermoset bakelite could be used a potential carbonaceous resource for reduction reactions. Key findings of this study are:
(1) Thermal degradation and structural evolution of bakelite was investigated in the temperature range 350–1450°C. Significant amounts of residual carbon were recovered after the heat treatment. Most of the weight loss occurred at temperatures below 700°C, and not much change later on at higher temperatures.
(2) Commercial bakelite generally contains significant levels (> 30%) of CaCO3 as filler. This proved to be highly beneficial for the utilization of bakelite in iron oxide composite pellets. In addition to acting as a fluxing agent, the decomposition of CaCO3 to CaO and the subsequent reaction of CaO with amorphous carbon led to the formation of CaC2 and the generation of graphitic carbon. Both graphitic carbon and CaC2 played an important role in enhancing the reaction kinetics of reduction reactions.
(3) Raw bakelite composite pellet showed 83.9% metallization degree at 1450°C after 20 minutes of reaction. The pellet retained its physical integrity after heat treatment and there was no clear separation between metal and slag. Small amounts of unreduced FeO were also observed. These partially reduced pellets could be smelted as direct reduced iron (DRI) in bath smelters.
(4) In Bakelite char based pellets; the metallization degree was found to be 90.12% after 20 minutes of reaction with the final product in the form of iron nuggets. These pellets showed a clear separation of slag and metal after heat treatment. The carbon pick-up in the reduced metal was determined as ~2.01 wt% which would have led to the localised melting of iron and its separation from slag.
(5) This study has shown that waste bakelite has the potential to be a valuable alternative carbon resource for ironmaking applications. It could be used either as raw bakelite or in the form of char to directly participate in iron oxide reduction reactions. The presence of impurities in the plastic waste did not have any detrimental influence as these were either precipitated out as slag or took part in reduction reactions. This work opens up novel avenues for recycling thermoset plastics that are generally hard to cycle and are often landfilled.