2014 Volume 54 Issue 4 Pages 715-720
2014 Volume 54 Issue 4 Pages 715-720
Serpentine was applied as a magnesium additive upon preparing oxidized pellets of a Brazilian hematite concentrate, the effects of ballability, preheating and roasting performance were studied in this work. The results showed that with the increasing dosage of serpentine, the green ball moisture content was increased, the compressive strength was improved too, but drop strength and burst temperature were reduced. In terms of the preheated pellets, the compressive strength were increased first and then decreased, and last leveled off. The compressive strength of roasted pellets were all improved, especially the improvement was obvious when the roasting was conducted at the temperature of 1280°C. When the serpentine ratio was constant, with the preheated temperature increasing, the compressive strength of preheated and roasted pellets were all improved. Mechanism analysis showed that the solid phase reaction could be promoted with addition of serpentine, and the consolidation strength of pellets would be increased also. Although the iron grade and reduction of pellets were reduced, the reducing swelling was decreased, but the softening begin temperature was risen, the softening range was narrowed, and the metallurgical performance of pellets was improved at the same time.
With the rapid development of the steel industry, and the depletion of the high quality magnetite concentrate both domestic and abroad. So many steel companies have to add poor quality domestic iron ore, but which contrary to the development direction of the blast furnace concentrate. Therefore, imbalance between iron ore supply and demand has become increasingly prominent,1) using hematite concentrate to produce pellets will become an option. The application of domestic hematite concentrate is problematic due to the low iron grade and coarse size which would require higher bentonite dosage during balling, and the addition of bentonite in turn would decrease the iron grade of the pellets. The high bentonite addition not only increases the cost of pellet production, but also increases the SiO2 content in the oxide pellet which lead to massive slag, and higher coke ratio of the blast furnace operation.2) Aiming to improve the pelletization of domestic iron ore, several studies have been conducted by the researchers. Lending of Brazilian hematite concentrate was proposed due to the higher iron grade, lower cost and finer size. However, the application of Brazilian hematite is limited by its poor ballability and high temperature performance. It is beneficial to the Chinese steel industry to produce pellets using Brazilian hematite concentrate at low energy consumption level and high yield, and this hematite is expected to be a new recourse which will help to tackle the shortage of iron ore supplement.3)
Since the new century, the demand of steel is being increased. At the same time, human being pay more and more attention to the living environment. Therefore, the steel enterprises pursue goals, including high-yield, low consumption, longevity and environmentally friendly.4) Blast furnace have certain requirements on the chemical composition of slag,5,6,7) when the slag basicity is 1.0–1.2, Al2O3 mass fraction is 13%–15%, MgO mass fraction is 10%–12%, the slag attains best performance, and blast furnace can achieve better smelting indicators.8,9,10,11) The MgO content of ore is insufficient to provide the slag required MgO, so usually the magnesium flux are added to the blast furnace. MgO plays important roles in the process of blast furnace smelting, containing improve slag reflow characteristics and slag stability, and so on. The magnesium flux are added directly to the blast furnace that is uneconomic and unscientific, because the main magnesium flux dolomite, magnesite, after being added to blast furnace, the carbonate will decomposition endotherm, to this end blast furnace coke will be risen.12,13,14) There researchers have explored boron additives, magnesium additive effects on magnetite pellets, Fan Guangquan15,16,17) thought that although the boron additive could reduce preheating and firing temperature, and increase preheated and roasted strength of the pellets, but it was not conducive to metallurgical properties. Zhang Yaping18,19,20) thought that the magnesium additive would improve preheated and indurated strength of pellets only at high preheating and roasting temperature, but could improve the metallurgical properties of magnetite pellets. The exploration and research of magnesium additives on the impact of hematite pellets, which is less in China.
Therefore, aiming at the defects of producing hematite concentrate pellets, the effects of serpentine on compressive strength of hematite pellets and metallurgical properties will be studied in this research, both good ballability performance, good mechanical properties and metallurgical properties hope to be attained, finally hematite will be fully utilized as pellet raw material.
The Brazilian import hematite are used in this work, as well as the self-serpentine. The chemical composition of materials are shown in Table 1.
| Name | TFe | FeO | SiO2 | Al2O3 | CaO | MgO | MnO | K2O | Na2O | S | P | LOI |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Brazilian hematite | 67.45 | 9.01 | 2.53 | 0.56 | 0.21 | 0.23 | 0.051 | 0.023 | 0.026 | 0.023 | 0.019 | 0.62 |
| Serpentine | 6.59 | 2.69 | 36.30 | 0.83 | 3.53 | 34.72 | – | 0.045 | 0.043 | 0.024 | 0.006 | 14.66 |
Table 1 shows that the iron grade of Brazilian hematite concentrate arrives at 67% or more, the SiO2 content is 2.53% as mainly gangue composition, the harmful impurities of S and P content are lower. The chemical analysis results indicate that the Brazilian hematite concentrate is high quality iron ore, and whose binary natural alkalinity is 0.083, belongs to an acidic mine. In addition, the FeO content of Brazilian hematite concentrate is low, at 9.01%, that can be speculated, ferric iron is mainly present in the form of hematite. MgO content of serpentine is high, at 34.72%, SiO2 content is high also, reaching 36.30%.
2.2. Iron Distribution of Hematite ConcentrateChemical phase method is used to test the iron distribution of the Brazilian hematite concentrate, according to the chemical solvent solubility and dissolution rate differences of every mineral or compound, the method of selective dissolution is used, the content of ferritic in various minerals or compounds are measured respectively, the results are shown in Table 2.
| Iron phase | Magnetite iron | Martite iron | Hematite (Limonite) iron | Carbonate iron | Sulfides iron | Silicate iron | Total |
|---|---|---|---|---|---|---|---|
| TFe (wt%) | 17.94 | 5.73 | 41.39 | 0.20 | 0.01 | 1.57 | 66.84 |
| Distribution (%) | 26.84 | 8.57 | 61.92 | 0.30 | 0.01 | 2.35 | 100.00 |
Table 2 shows that the iron of Brazilian hematite concentrate exists mainly in hematite (limonite) and martite, both total iron is 47.02%, following by the magnetic iron, the iron content is 17.94%, and other ferrous iron content material phase are minimal. It can be seen clearly, Brazilian hematite concentrate exists mainly in the form of hematite (limonite).
2.3. Size Distribution of Raw MaterialsUsing grain size to calculate the size composition of raw materials. The specific surface is measured referring to Blaine method (GB8074-87), testing in Blaine instrument. The principle is based on when a certain amount of air through a certain porosity and a constant thickness layer of powder material, the flow rate of changing is caused by the different resistance of air, and the specific surface area of materials is measured. The results are shown in Table 3.
| Raw material | Particle size range | specific surface area (cm2.g–1) | ||
|---|---|---|---|---|
| –0.074 mm | –0.074~–0.045 mm | –0.045 mm | ||
| Brazilian hematite | 1.17 | 3.11 | 95.72 | 1647.00 |
Table 3 shows that the –0.074 mm grain size content is 98.83%, and the –0.045 mm grain size content is 95.72%. In terms of particle size, the hematite reaches a size requirements of raw materials pelletizing process. The specific surface area of hematite concentrate is 1647.00 cm2·g–1, according pellet production experience, the hematite reaches the requirements of a specific surface area of raw materials pelletizing process.
2.4. Physical Properties of BentoniteBentonite is widely used and a good binder in the production oxide pellets. Montmorillonite is the main mineral composition, besides bentonite contains a variable number of clay minerals (such as kaolinite, etc.) and non-clay minerals (such as quartz, feldspar, etc.). Montmorillonite belongs to a layered structure of aluminum silicate minerals, with strong absorption, good expansion, and thus can improve green pellet strength and dry pellet strength (especially drop strength of green ball), can adjust pelletizing water and stable pelletizing process simultaneously.
The chemical composition of bentonite is mainly SiO2 and Al2O3, also contains a certain amount of K2O and Na2O, and small amounts of CaO, MgO, Fe2O3, etc. So high dosage of bentonite will reduce the pellets iron grade, but too high K2O, Na2O will affect the blast furnace adversely.
The main chemical composition of bentonite are shown in Table 4. The main physical properties are shown in Table 5.
| Name | TFe | SiO2 | Al2O3 | CaO | MgO | K2O | Na2O | FeO | LOI |
|---|---|---|---|---|---|---|---|---|---|
| Bentonite | 1.94 | 63.44 | 14.55 | 2.38 | 3.63 | 0.32 | 2.13 | 0.15 | 10.03 |
| Name | Moisture (%) | Glial price (%/(3 g)) | Capacity expansion (ml/g) | Absorption (%) | Blue absorption (g/100 g) | Montmorillonite content (%) | –0.074 mm (%) |
|---|---|---|---|---|---|---|---|
| Bentonite | 12.665 | 100 | 15.0 | 436.01 | 27.5 | 62.22 | 99.4 |
Pelletizing test carries out in the disc pelletizer, the main technical parameters: Disc diameter ψ =1000 mm, speed 22 rpm, side height h=150 mm, inclination angle α=47°. The green pellet moisture content is 9.5% in pelletizing trials, the pelletizing time is 12 min, bentonite dosage is 0.8%. Preheating test conductes in a dry cup, roasting test carries out in a small rotary kiln shown in Fig. 1. The compressive strength of pellets is measured by intelligent pellet compression tester, and analyzed the chemical composition of finished pellets. Pellets metallurgical properties includes reduction, reducing swelling, reduction degradation and soft melting performance. Reduction is measured by balance weight, GB/T13241-91, reducing swelling index (relatively free swelling index) is determined by GB/T13240-91 standards, reduction degradation is determined by GB/T13242-91, the temperature method is adopted to measure reflow performance.
The devices of dry cup and rotary kiln in laboratory.
The mixture is made to ball according to the Table 6 ratio, and the balling time is 12 min, as follows.
| No. | Hematite concentrate | Bentonite | Moisture | Serpentine | Total |
|---|---|---|---|---|---|
| 1 | 89.70 | 0.80 | 9.50 | 0.00 | 100 |
| 2 | 88.32 | 0.80 | 9.50 | 1.38 | 100 |
| 3 | 86.95 | 0.80 | 9.50 | 2.75 | 100 |
| 4 | 85.57 | 0.80 | 9.50 | 4.13 | 100 |
| 5 | 84.20 | 0.80 | 9.50 | 5.50 | 100 |
Research effect of serpentine on property of green pellet, the mixture ratio accords to the Table 6, and pelletizing time is 12 min, when serpentine dosage is different, drop strength, compressive strength and burst temperature of green balls will be studied mainly. The results are shown in Fig. 2.

Effect of serpentine on property of green pellet.
Figure 2 shows that moisture content of the green balls is increased with addition of serpentine, because MgO of the serpentine has better absorbent than iron ore. The compressive strength of green ball is improved, the increase is greater than the moisture of green ball, while the drop strength and burst temperature are decreased, but changed slightly.
3.2. Effect of Serpentine on Preheated Ball Performance 3.2.1. Effect of Serpentine Dosage on Different Preheating Temperature of Preheated Pellets Compressive StrengthThe mixture ratio accords to the Table 6, and 12 min balling time, when preheating time is 8 min, the results will be indicated as Fig. 3. Through reviewing the effects of varying preheating temperature upon strength of preheated pellets with different serpentine dosage.

Effect of serpentine dosage on different preheating temperature of preheated pellets compressive strength.
The Fig. 3 shows, when preheating temperature is constant, the trend of compressive strength of preheated pellets reflects first and then down with the increase of serpentine addition, which means when serpentine dosage increases from 0% to 1.38%, the compressive strength of preheated pellets will be increased as well, and continuing to increase serpentine dosage, the compressive strength of preheated pellets begins to decrease drastically, and then becomes flat, and the general compressive strength of preheated pellet is comparatively low. While the serpentine dosage is fixed, the compressive strength of preheated pellets reflects rising trend, but only when the preheating temperature is increased over 1000°C, the compressive strength of preheated pellets can reach above 400 N.
3.2.2. Effect of Serpentine Dosage on Different Preheating Time of Preheated Pellets Compressive StrengthThe mixture ratio accords to the Table 6, and 12 min pelletizing time, when preheating temperature is 1000°C, the results will be indicated as Fig. 4. Through reviewing the effects of varying preheating temperature upon strength of preheated pellets with different serpentine dosage.
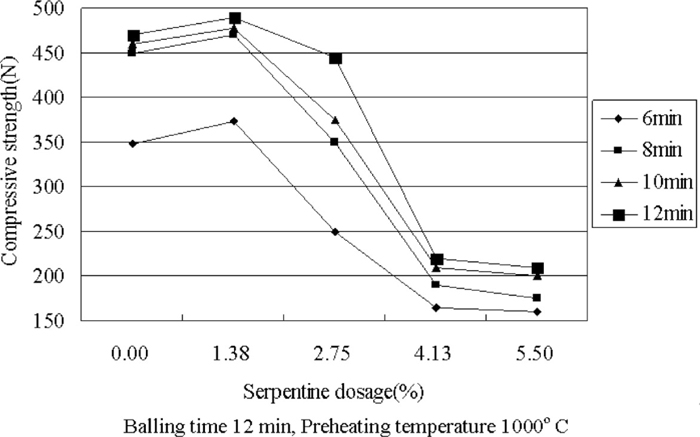
Effect of serpentine dosage on different preheating time of preheated pellets compressive strength.
When preheating time is constant, the compressive strength of preheated pellets will rise a little when serpentine dosage increases to 1.38%, but with continuing to increase serpentine dosage to 4.13%, the compressive strength of preheated pellets will be decreased drastically in this process, and when serpentine dosage increases to 5.5%, the compressive strength of preheated pellet will keep the same. When serpentine dosage is constant, the compressive strength of preheated pellets will reflect rising trend, and the increase of compressive strength will reach maximum during prolonging the preheating time from 6 min to 8 min.
The effects of preheating temperature and preheating time on compressive strength with different serpent dosage show that, given the economic characteristics of pellet production, considering the best preheating temperature is 1000°C, and the best preheating time is 8 min, and the value of compressive strength of preheated pellets will be optima when serpentine dosage is 1.38%.
3.3. Effect of Serpentine on Indurated PelletsAs studying the effect of preheating system on compressive strength of preheated pellets with different serpentine proportions, on the condition of 0.8% bentonite, 9.5% balling moisture, and 12 min pelletizing time, to review the effect of indurating time and roasting temperature on compressive strength of fired pellets with different serpent dosage while preheating temperature is constant at 1000°C, and preheating time is 8 min.
3.3.1. Effect of Serpentine Dosage on Different Roasting Time of Indurated Pellets Compressive StrengthOn the condition that indurating temperature is 1250°C, effects of roasting time on compressive strength of indurated pellets with different serpent dosage will be showed as Fig. 5.

Effect of serpentine dosage on different roasting time of indurated pellets compressive strength.
The Fig. 5 shows, when indurating time is constant, and serpentine dosage is increased from 0% to 2.75%, the range of compressive strength of indurated pellets will be increased a lot, while serpentine dosage is increased from 2.75% to 5.5%, the increase range of indurated pellets will become small. When serpentine dosage is above 2.75%, and compressive strength of indurated pellets all reach 3000 N. When the serpentine proportion is constant, with the prolonging of indurating time from 9 min to 12 min, the increase range of compressive strength of indurated pellets will be maximum. When indurating time is 15 min and 18 min, then value of compressive strength will reflect a state of crossover.
3.3.2. Effect of Serpentine Dosage on Different Roasting Temperature of Indurated Pellets Compressive StrengthThe effects of indurating temperature on compressive strength of indurated pellets with different serpentine proportions will be showed as Fig. 6 when the indurating time is 12 min.

Effect of serpentine dosage on different roasting temperature of indurated pellets compressive strength.
The Fig. 6 shows, when indurating temperature is constant, with the increase of serpentine dosage, the compressive strength of roasted pellets will be enhanced, and when indurating temperature is 1280°C, change of the curve becomes the most obviously. When temperature lies between 1250°C–1280°C, the increase range of compressive strength of indurated pellets is small, but with continuing to increase indurating temperature, compressive strength of indurated pellets will decrease first and then increase drastically. In addition, compared with indurated pellets without adding serpentine, there found that the serpentine can improve compressive strength of indurated pellets, and the more the serpentine dosage, the higher the compressive strength of indurated pellets.
As learned from the effects of indurating time and firing temperature on compressive strength of indurated pellets with different serpentine dosage, the optimal indurating time is 12 min with optimal indurating temperature 1280°C.
3.4. Optimization TestBasing on the serpentine effects the compressive strength and reducing swelling of hematite pellet, the compressive strength and metallurgical properties of pellets will be studied, optimization test will be further carried out, the test conditions are shown in Table 7. Comprehensive comparing without adding serpentine and with addition of 5.5%, the chemical composition of pellets are shown in Table 8 and the metallurgical properties pellets are shown in Table 9.
| No. | Hematite concentrate (wt%) | Bentonite (wt%) | Moisture (wt%) | Serpentine (wt%) | Balling time (min) | Preheating temperature (°C) | Preheating time (min) | Roasting temperature (°C) | Roasting time (min) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 89.70 | 0.80 | 9.50 | 0.00 | 12 | 1000 | 8 | 1280 | 12 |
| 2 | 84.20 | 0.80 | 9.50 | 5.50 | 12 | 1000 | 8 | 1280 | 12 |
| serpentine | TFe | FeO | SiO2 | Al2O3 | CaO | MgO | K2O | Na2O | S | P |
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 65.38 | 0.46 | 4.10 | 0.45 | 0.27 | 0.24 | 0.04 | 0.035 | 0.014 | 0.025 |
| 5.50 | 63.29 | 1.08 | 4.40 | 0.67 | 0.45 | 1.95 | – | – | 0.017 | 0.021 |
| serpentine (%) | Reduction degradation index (%) | Reduction degree RI (%) | Restore expansion RSI (%) | Softening begin temperature T10 (°C) | Softening end temperature T40 (°C) | Softening range ΔT (°C) | |
|---|---|---|---|---|---|---|---|
| RDI+6.3 | RDI–0.5 | ||||||
| 0 | 96.50 | 1.50 | 73.56 | 10.07 | 1073 | 1148 | 75 |
| 5.50 | 96.10 | 1.90 | 71.88 | 8.08 | 1084 | 1151 | 67 |
Table 8 shows that the TFe of pellets is 63.29% with addition of serpentine, which is lower 2.09% than without adding serpentine. The FeO content of pellets is 1.08%, which is higher 0.62% than without adding serpentine. The content of harmful elements S and P are considerable, the content of MgO is 1.95%, which is higher 1.71% than without adding serpentine, the content of SiO2 is nearly.
Table 9 indicates that the RDI of pellets is reduced with addition of serpentine, RDI+6.3 is reduced to 96.10% from 96.50%, because RDI–0.5 is increased to 1.90% from 1.50%. RI of pellets is 71.88%, which is lower 1.68% than without adding serpentine. RSI is 8.08%, which is lower 1.99% than without adding serpentine. Softening begin temperature is enhanced 11°C and softening end temperature is risen 3°C, softening range is narrowed 8°C.
3.5. Mechanism AnalysisThe optimization test is carried out mineralogical microstructure research, Figs. 7 and 8.
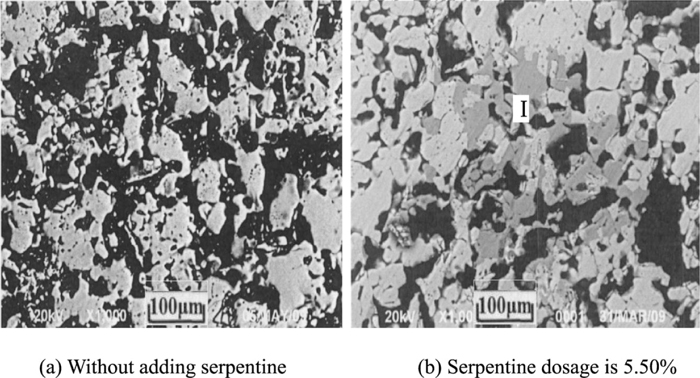
The SEM micrographs of roasted pellets.
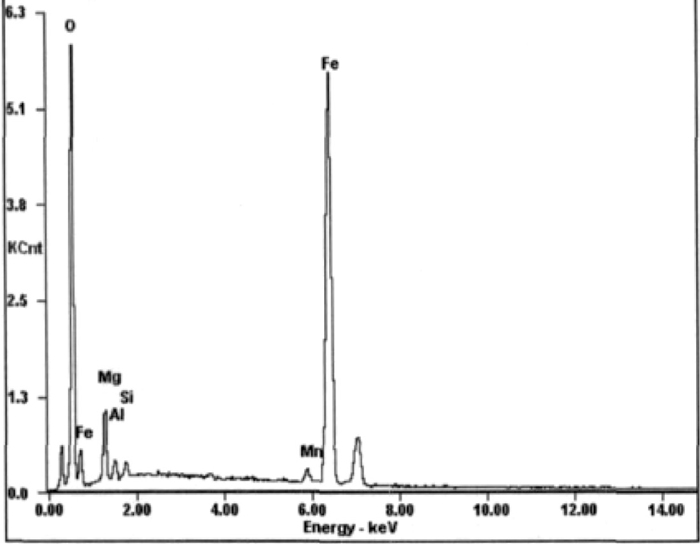
The SEM energy spectrum of MgO solid solution with hematite.
Figure 7(a) indicates that Fe2O3 particles crystalline of pellets is small without adding serpentine, only a small portion of recrystallization begin interconnection. Most Fe2O3 are single granular, present initial crystalline, particle angular are obviously, between the particles is almost no liquid to fill, thereby many irregular shape size of the pores will be formed. It can be seen, in the roasted process, although some Fe2O3 recrystallization, but there is no liquid phase formed, so consolidation can not be promoted effectively. Most Fe2O3 present initial crystalline yet, because of consolidation does not close between the particles, which will cause large voids between the particles, density level and compressive strength of pellet are low.
Figure 7(b) demonstrates that hematite grain growth and recrystallization take place with adding serpentine, and many new particles are generated within pellets, which are similar with hematite, however, these particles color are yellowish gray under reflective and are intertwined with hematite. These new particles will be scanned Fig. 8, and found that these new particles are not magnesium ferrite, instead MgO solid solution with hematite (I). Studies have shown that, Mg2+ can play a stabilizing role in hematite lattice. The consolidation of pellets depend on solid phase consolidation mainly, through the solid particle diffusion reaction to form connecting bridges (or connection neck), particles will be bonded together depending on compounds or solid solution, thereby the strength of pellets consolidation will be enhanced. Thus, solid phase reaction can be promoted through adding serpentine, the consolidation strength of pellets will be improved.
Analyzing from the impact trend, after adding serpentine, the grade iron of finished pellets and reduction degree are decreased, reducing swelling is reduced, the soften beginning temperature is risen, softening range is narrowed, high temperature metallurgical properties of pellets will be improved. Serpentine helps to improve soft melt and drip performance of acidic pellets. The reason is that: MgO can promote the generation of high-melting slag phase, and inhibit the formation of low melting iron silicate, which cause the melting temperature to rise. In the heating process, MgO and FeO occur an infinite solid solution, which will form a high melting point of magnesium wustite, so that the droplet temperature is risen.
(1) With addition of serpentine, the increase of serpentine dosage, moisture of green balls is increased, the compressive strength of green balls is improved also, while drop strength and burst temperature are decreased reversely.
(2) Whether preheating temperature and preheating time are constant, with increasing of the serpentine dosage, the compressive strength of preheated pellets is increased first and then decreased, hereafter is showing gently. When the addition of serpentine is constant, with the preheating temperature changing, the compressive strength of preheated pellets reflect upward trend all, only when the preheated temperature is rised above 1000°C, the compressive strength of preheated pellets will be to achieve per 400 N. With the extension of preheating time, compressive strength of preheated pellets all reflect upward trend, only in the process of the preheating time extends from 6 min to 8 min, compressive strength of preheated pellets will be increased the most.
(3) When roasting time and firing temperature are constant, with increasing of the serpentine dosage, the compressive strength of roasted pellets are increased, but when the roasting temperature is 1280°C, the compressive strength of indurated pellets is higher, the variation curve is most obviously. When serpentine dosage is constant, with the extension of roasting time, the time extends from 9 min to 12 min, the compressive strength of roasting pellets are highest. Comparing with no addition of serpentine, there found that serpentine can increase the compressive strength of roasted pellets, with serpentine dosage increasing, the compressive strength of roasted pellets are also on the rise.
(4) With addition of serpentine, serpentine has prompted solid phase reaction occurs, can improve the consolidation strength of pellets. The grade iron of finished pellets and reduction degree are decreased, reducing swelling is reduced, the soften beginning temperature is rised, softening range is narrowed, at the same time high temperature metallurgical properties of pellets will be improved.