2014 Volume 54 Issue 8 Pages 1935-1942
2014 Volume 54 Issue 8 Pages 1935-1942
Traditional techniques and methods can not resolve the contradictions of the microstructure requirement between creep resistance and oxidation resistance in T91 steel at high temperature. In the present work, oxidation resistance of T91 steel were successfully improved by surface mechanical attrition treatment (SMAT). The variations of ferrite grains and precipitation behavior in the surface layer at different tempering temperature were also observed to investigate thermal stability. A FeCr2O4 spinel inner layer with higher Cr content could rapidly form and protect the material from further oxidation in T91 steel due to the nanocrystalline surface layer.
T91 steel is considered as one of the ideal structural materials for steam pipes, high temperature boilers and turbine parts in advanced fossil fired power plants.1) Long-term exposures of T91 steel at high temperature would lead to oxidation. Alloying with Cr at an amout of more than 10 wt.% can protect the material against oxidation by forming chromia. One of the critical issues for the components of ferritic heat resistance steels is ensuring sufficient creep strength under the operation conditions. However, when the Cr content surpass 10 wt%, creep properties would decrease due to the appearance of δ ferrite.2) Traditional metallurgical techniques and methods can not resolve the contradictions of the Cr content requirement between creep resistance and oxidation resistance in T91 steel at high temperature.
Nanocrystalline materials have attracted intensive interest due to the significance in both mechanical properties and physical properties.3) Surface mechanical attrition treatment (SMAT) is a newly developed surface modification technique by severe plastic deformation to generate a thin skin of nanostructures on alloys. The effect of nanocrystalline or ultrafine grains on wear resistance,4) corrosion behavior, fatigue behavior,5) and mechanical properties6) has been studied in ferritic steels. The results indicate that mechanical properties and physical properties are significantly improved. The diffusivity of Cr in nanocrystalline Fe is 7-9 orders of magnitude higher than that in Fe lattice, and it is 4-5 orders of magnitude higher than that in normal grain boundaries.7) The mean size of ferrite grains and M23C6 particles at the top surface is about 8 and 4 nm, respectively, they increase gradually with increasing depth in nanocrystalline ferritic steels.7,8,9) An important factor to form protective spinel (Fe, Cr)3O4 layer is an increase of Cr diffusivity in T91 steel. In general, the finer grain size and more deformation amount are adopted to increase the density of fast diffusion paths. Provided creep properties have been improved by controlling the microstructure, SMAT is selected to produce surface nanocrystalline layer, which could achieve the improvement of the high temperature oxidation resistance.
The chemical composition of T91 steel is shown in Table 1. Prior heat treatment is austenitizing at 1253 K for 45 min, water quenching (WQ). A plate specimen (100 × 50 × 4.0 mm3 in size) of the quenched steel was subjected to SMAT. The set-up and procedure were described as follows: about 50 spherical steel balls were placed in a reflecting chamber that was vibrated by a vibration generator. The hardened 0.8C steel balls were 5 mm in diameter with a mirror-like surface. The vibration frequency of the chamber was 20 kHz. Once the balls were resonated, a large number of flying balls would impact the specimen surface. Consequently, the repeated multidirectional impacts at high strain rates onto the specimen surface would result in severe plastic deformation and grain refinement progressively down to the nanometer regime in the entire specimen surface. After SMAT with a duration of 30 min, the surface roughness is comparable to that of the original specimen. The other experimental setup and the details of SMAT processing were described in the reference.9)
| C | Si | Mn | Cr | Mo | Ni | V | Nb | N | S | P | O | Fe |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.10 | 0.36 | 0.60 | 8.80 | 0.95 | 0.23 | 0.20 | 0.08 | 0.04 | 0.0017 | <0.005 | <0.002 | BaL |
After the nanocrystalline microstructure was induced by SMAT, the specimes were tempered at 893 K and 1033 K for different holding times (1.5 h, 3 h, 10 h, 16 h, 30 h) in glass tubes with argon/dry air, and the change of microstructure and oxide scale were determined by OM, SEM, TEM and XRD. XRD experiments were performed on a Rigaku D/max 2400 X-ray diffractometer (12 kW) with Cu Kα radiation. The average crystallite size in the surface layer produced by SMAT was derived from the breadths at half maximum intensity of measured Bragg diffraction peaks using the Scherrer-Wilson method. The cross-sectional morphologies of specimens were observed by using a JEM-6301F and TESCAN-VEGA TS5136XM SEM with back-scattered electron (BSE) and EDX. Microstructure characterizations of nanocrystalline microstructure were also examined by using a JEM 200CX transmission electron microscope (TEM). Thin foil specimens for TEM observation were prepared by using ionmilling at low temperatures.
Optical microscope images Fig. 1(a) and SEM images Fig. 2(a) show effect of SMAT on ferrite grains in quenched T91 steel, respectively. The nanocrystalline surface layer is obtained in the specimen treated by SMAT. Owing to the gradient variation of the strain and strain rate from the treated top surface (both are very large) to the deep matrix. Severe plastic deformation layer denotes where the coarse-grained structure in the surface layer is refined into the micrometer–nanometer regime. A gradient grain size distribution from a few nanometers (in the top surface layer) to several micrometers is developed in the surface of SMAT sample. Total plastic deformed layer includes the deformed coarse-grain layer. Schematic illustration of microstructure characteristics were also described in the reference.9) Cross-sectional morphologies (Figs. 1 and 2) show the clear evidence of severe plastic deformation with 100 μm thick in the specimen, and the total plastic deformed layer is about 260 μm thick. Owing to the plastic deformation in the surface layer induced by SMAT, the coarse-grained structure in the surface layer is refined into the nanometer scale. The mean size of ferrite grains is 10 nm in the top surface of T91 steel treated by SMAT as shown in Fig. 3. The mean size of ferrite grains increases to be 50 nm in the top nanocrystalline surface layer after tempering at 893 K for 90 min, and ferrite grains size is still unchanged with increasing tempering time to 30 h as shown in Fig. 1(d). In the meantime, there are some finer Cr-rich carbides in the top surface layer as shown in Fig. 2(b), the mean size of carbides is little variation in the different position of the specimen after tempering at 893 K.

Cross-sectional OM observations of microstructure in (a) the quenched T91 steel treated by SMAT; (b) the specimen treated by SMAT and tempered at 893 K for 90 min; (c) the specimen treated by SMAT and tempered at 1033 K for 90 min; (d) the specimen treated by SMAT and tempered at 893 K for 30 h.
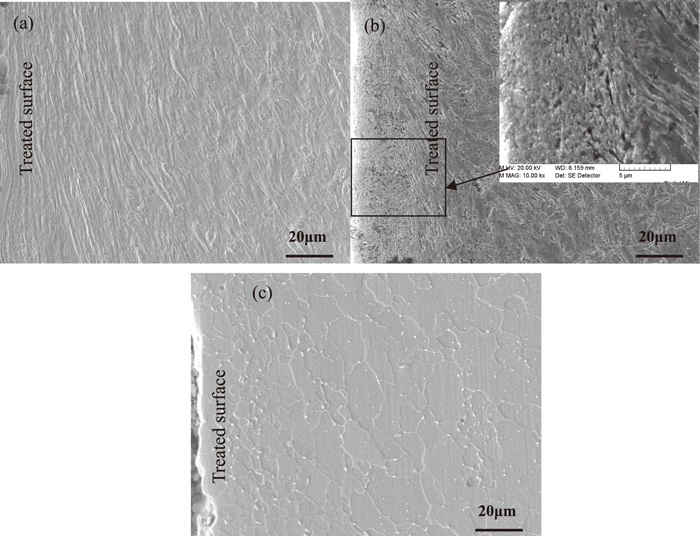
Cross-sectional SEM observations of microstructure (a) the quenched T91 steel treated by SMAT; (b) the specimen treated by SMAT and tempered at 893 K for 90 min; (c) the specimen treated by SMAT and tempered at 1033 K for 90 min.

Cross-sectional SEM observations and TEM observations of different micristrucutre in quenched T91 steel treated by SMAT (a) nanocrystalline microstructure of the top surface; (b) the subsurface; (c) the un-deformed matrix; (d) nanocrystalline microstructure of the top surface.
The average grain/cell size of ferrite along depth in the surface layer gradually increases in the specimens, the results at different tempering temperature are shown in Figs. 1 and 2. There are fine equiaxed crystal with mean size of 2 μm, coarse equiaxed crystal with 20 μm and coarse columnar crystal with average dimensions of 40 μm along the short axis and 80 μm along the long axis in the specimen after tempering at 1033 K for 90 min. The ferrite grain is still fine in the top surface, the growth occurs in the region of 120–280 μm deep from top surface as shown in Figs. 1(c) and 2(c). Figure 3 indicates Cross-sectional observations of the different positions along the depth in the surface layer of quenched T91 steel treated by SMAT. The microstrain decreases significantly along the depth, and the mean size of ferrite grains is refined to be 8 nm in the top surface layer of T92 steel treated by SMAT [9], this result is well in agreement with the T91 steel treated by SMAT. The average size change of ferrite grains/cells in those specimens is comparable to the quenched specimen treated by SMAT, only the recovery of ferrite grains occurs during tempering at 893 K in Fig. 4. The mean size of Cr-rich carbides is little variation in the different position of the specimen tempered at 893 K. It may be difficult to distinguish the tiny change according to SEM results. M23C6 (M = Cr, W, Fe) carbides precipitate during tempering at 1033 K, and smaller grains and coarser M23C6 carbides are observed in the top surface layer than that of the un-deformed matrix, as shown in Fig. 5. The mean size of carbides almost increases to 1 μm in the top surface layer of the specimen treated by SMAT after tempering at 1033 K for 90 min, then decreases slightly in the severely deformed layer and matrix. The mean grain size of ferrite is determined to be about 3 μm in the top surface layer of the specimen treated by SMAT after tempering at 1033 K for 30 h. It could be inferred that the nanocrystalline surface layer is quite stable until 1033 K. Thermodynamically, as a mainly part of driving force for grain growth, the grain boundary energy is regarded as constance in common. However, under the condition of solute segregation in GBs, on the basis of Gibbs adsorption theory, the GB energy is thought to be reduced with segregation promoting in nanocrystalline produced by SMAT. Furthermore, a systemic metastable state where the GB energy reduces to zero with solute segregating. Namely, the GB segregation reduces the driving force to retard grain growth. The recrystallization kinetics originates from numerous nanocrystalline ferrite grain boundaries with a high excess stored energy in the surface layer due to severe plastic deformation during SMAT. Howerve, it do not meet the drive force of recrystallization at 1033 K. So the nanocrystalline surface layerof T91 steel is quite stable until 1033 K.

Cross-sectional SEM observations of different micristrucutre in quenched T91 steel treated by SMAT after tempering at 893 K for 90 min (a) nanocrystalline microstructure of the top surface; (b) the subsurface; (c) the un-deformed matrix.

Cross-sectional SEM observations of different micristrucutre in quenched T91 steel treated by SMAT after tempering at 1033 K for 90 min (a) nanocrystalline microstructure of the top surface; (b) the subsurface; (c) the un-deformed matrix.
The average size of ferrite grains and M23C6 carbides as a function of depth in both the specimens is measured by using a series of analysis techniques, as summarized in Fig. 6. It is clear that the surface layer in T91 steel treated by SMAT is nanocrystalline and the average grain size increases with increasing depth. The growth of ferritic grains occurs in the surface layer of T91 steel treated by SMAT after tempering at 1033 K.

Schematic illustration of mean size in ferrite grain and M23C6 carbides as a function of depth in both the quenched sample treated by SMAT and the sample after tempering at 1033 K for 90 min.
T91 steel with nanocrystalline microstructure rapidly formed a FeCr2O4 inner oxide and Fe2O3 + Fe3O4 outer oxide after tempering at 893 K and 1033 K by SEM and XRD analysis. For the “inner” and “outer” oxide layers in T91 steel, there are different chemical elements and phase, an inner scale layer (usually fine-grained) consisting of Fe–Cr spinel oxides, and an outer layer which has a columnar-grained structure, and consists of essentially Cr-free magnetite (Fe3O4). Discontinuous patches of haematite were visible in the outer surface of the oxide, and separation had occurred between the inner and outer layers. Semi-quantitative linescan chemical analysis of oxide scale of the specimen after tempering at different temperature indicates that the outer layer consists of Fe and O, and so is Fe2O3. In contrast, the inner oxide layer is enriched in the element Cr compared to the alloy and outmost layer, suggesting that the inner layer is to be protective spinel FeCr2O4 oxide in Figs. 7 and 8. Cross section of the scale in the specimen after tempering at 893 K for 90 min, shown in Fig. 7(a), show them to consist of a Fe2O3 layer of approximately equal thickness and a dark appearing line (typically Cr-rich 14 wt.%). Cross sections of the scale in the specimen after tempering at 1033 K for 90 min, as shown in Fig. 8, showing them to consist of the Fe2O3 outer layers and the spinel FeCr2O4 inner layer enriched in Cr with about 16 wt.% based on SEM and XRD results. However, the FeCr2O4 layers are not detected in the specimen without nanocrystalline microstructure, and the most scale is discontinuous, as shown in 8(b). The thickness of both oxide inner layer and outer oxide layer is smaller in nanocrystalline surface at 1033 K for 30 h as shown in Fig. 9. The growth rate of scale at 1033 K was plotted in Fig. 10. It can be inferred that the growth rate decreased in T91 steel with nanocrystalline surface layer.

Cross-sectional BSE observations of oxide scales formed at 893 K for 90 min (a) quenched T91 steel treated by SMAT; (b) quenched T91 steel; (c) the results of EDS linescan corresponding to figure (a).

Cross-sectional BSE observations, EDX linescan and XRD analysis of oxide scales formed at 1033 K for 90 min in (a), (c), (e) quenched T91 steel treated by SMAT; (b), (d), (f) quenched T91 steel.

Cross-sectional BSE observations of oxide scales formed in T91 steel treated by SMAT (a) and T91 steel (b) at 1033 K for 30 h.

Effect of SMAT processes on growth rate of the inner and outer layers at 1033 K.
Ferrite grains after deformation by SMAT may affect the recrystallization during tempering. Grain boundaries are good sites for nuclei to form. A decrease in prior grain size will result in more boundaries, which cause an increase in nucleation rate and a decrease in recrystallized grain size. The recrystallization kinetics originates from numerous nanocrystalline ferrite grain boundaries with a high excess stored energy in the surface layer due to severe plastic deformation during SMAT. Generally, the deformation increases the rate of nucleation faster than the rate of growth. As a result, the final grain size reduces by increasing deformation & annealing as shown in Figs. 1(b) and 2(b). The growth rate of recrystallization grains10) could be expressed as Eq. (1).
| (1) |
The mean size of recrystallization grains11) also could be expressed as Eq. (2)
| (2) |
Strain of some grains would decrease to a certain extent with increasing the depth from the top surface layer, and the nucleation rate would not increase. The deformed ferrite grains are characterized by high dislocation densities and high stored energy ES, so the value of G/N is very large due to an increase of G in the region of 120–280 μm deep from top surface. Therefore, the change of the grain size of ferrite along the depth of the specimen treated by SMAT after tempering at 1033 K would be sound, as shown in Figs. 1(c) and 2(c).
M23C6 (M = Cr, W, Fe) carbides precipitate in T91 steel treated by SMAT during tempering at 1033 K, and the carbides in the top surface layer are far larger than that of the un-deformed specimen, as shown in Fig. 5. M23C6 carbides with the mean size of 150 nm mainly precipitate along prior austenite grain and packet boundaries.11) However, the mean size of carbides almost increases to 1 μm in the surface layer of the specimen.
The growth of M23C6 is controlled by the diffusion of the substitutional component chromium in 9Cr reduced activation steels.11,12) According to the LSW theory of Ostwald Ripening13) and the work of Brailsford et al.,14,15) the mean size of M23C6 would be express as Eq. (3)
| (3) |
The coarse-grained structure in the surface layer is refined into the nanometer scale by SMAT without any change of the chemical compositions,9) so the volume fraction of M23C6 carbides and the coefficient of coarsening rate Pf should be invariable. Effect of the increase in interface energy σ on coarsening kinetics of M23C6 can be neglected, because mismatch of atomic arrangement are similar in both the specimen treated by SMAT and the un-deformed specimen, only plastic deformation induces more interfaces and dislocations. The carbon and chromium content in ferrite is invariable in T91 steel treated by SMAT,8) so it could be concluded that the coarsening kinetics of M23C6 only depends on the diffusion coefficient of chromium in ferrite. The effective diffusivity in the severely deformed surface layer is more than 2 orders of magnitude higher than that along conventional high-angle grain boundaries in Cu16) and Ni.17) Wang et al.7) report that the diffusivity of Cr in the SMAT-induced nanocrystalline Fe is 7-9 orders of magnitude larger than that in Fe lattice, and 4-5 orders of magnitude higher than that in the Fe grain boundaries.18,19) According to the Eq. (3), the diffusion rate of chromium along nanocrystalline grain boundaries is approximately 300 times than that of the coarse grain boundaries. Therefore, it could be refered that refining the grains in the surface layer of T91 steel treated by SMAT to enhance the chromium diffusion is one of reasons that cause the nearly an order of magnitude increase of mean size of M23C6 carbides.
The typical scales are found to be double-layered as Fe2O3/Fe3O4 and Fe–Cr spinel layer in T91 steel, also, the inner Fe–Cr spinel layer has often been observed to contain multiple, partial layers of Cr-rich oxide.20) Linescan chemical analyses of oxide scale of the SMAT specimen after tempering at different temperature indicates that the outer layer consists of Fe, O (essentially hematite), and the inner layer enrich in Cr is spinel layer, suggesting that with nner layer of the specimen is more protective than the specimen untreated by SMAT. The oxidation reaction is assumed to be purely controlled by the diffusion of chromium. The Cr-diffusion distance (x) after a duration (t) can be described by Fick’s second law:
| (4) |
Most commercial high-temperature alloys are designed to contain sufficient Cr so that they can form Cr2O3 scales. But Cr dissolves in magnetite (Fe3O4) to form Fe–Cr spinel-type oxides in steels. The Fe–Cr spinels have variable stoichiometry (FeFe2–xCrxO4), which can accommodate varying levels of Cr without large changes in structure, and exhibit reduced diffusion of iron ions with increasing Cr content.20)
The diffusivity of Cr in the nanocrystalline Fe is 4-5 orders of magnitude higher than that in the normal grain boundaries,3,4,5,6,7,8,16,17,18) the diffusivity of oxygen inwards to the alloy-oxide interface and the diffusivity of iron outward to the air-oxide interface extremely increases as well in the nanocrystalline T91 steel. Therefore, T91 steel with nanocrystalline microstructure rapidly forms the double layers as Fe2O3 and FeCr2O4, and this will lead to acceleration of the oxidation rate in the early stage. On Fe–Cr alloys oxidized in air, the increase of Cr content in the inner layer has the effect of reducing the diffusion rate of iron ions outwards and oxygen ions inwards. Cr content of the inner layer in the nanocrystalline surface is large than that of the undeformed surface, as shown in Table 2. It is the main reason that the rate of thickening of the outer oxide layers decreases after exposure at 1033 K for 10 h. T91 steel with nanocrystalline microstructure rapidly forms a FeCr2O4 oxide with sufficient thick that protects the material against further oxidation. The inner FeCr2O4 layer with high Cr content in the specimen after tempering at 1033 K for 1.5 h is about 10 μm. The results demonstrate the effectiveness of nanocrystalline boundaries as preferential diffusion paths for Cr, hence increase the flux of Cr to the alloy surface and then enhance the development of an internal Fe–Cr spinel scale. The increase of FeCr2O4 spinel scale to a certain extent with increasing time can lead to reduction of the diffusion rate of iron ions outwards and oxygen ions inwards, so that the rate of thickening of the oxide layers decreases.
| O | Fe | Cr | Cr/Fe | |
|---|---|---|---|---|
| un-deformed specimen + 1033 K × 1.5 h | 25 | 61 | 13 | 0.21 |
| specimen produced by SMAT + 1033 K × 1.5 h | 24 | 55 | 16 | 0.29 |
| un-deformed specimen + 1033 K × 3 h | 25 | 58 | 13 | 0.22 |
| specimen produced by SMAT + 1033 K × 3 h | 25 | 57 | 17 | 0.30 |
| un-deformed specimen + 1033 K × 10 h | 30 | 51 | 11 | 0.21 |
| specimen produced by SMAT + 1033 K × 10 h | 24 | 54 | 17 | 0.31 |
| un-deformed specimen + 1033 K × 16 h | 26 | 53 | 12 | 0.22 |
| specimen produced by SMAT + 1033 K × 16 h | 23 | 54 | 17 | 0.31 |
| un-deformed specimen + 1033 K × 30 h | 24 | 60 | 12 | 0.20 |
| specimen produced by SMAT + 1033 K × 30 h | 26 | 54 | 16 | 0.30 |
The change of microstructure and oxide scale in quenched T91 steel with nanocrystalline microstructure induced by SMAT are studied at different tempering temperature. The thermal stability of nanocrystalline microstructure is excellent in T91 steel until the tempering temperature hits 893 K. The smaller grains and coarser M23C6 carbides are observed in the top nanocrystalline surface layer after tempering at 1033 K than that of the un-deformed matrix. The increase of mean size in M23C6 carbides depends on the higher Cr diffusivity in the nanocrystalline surface layer of T91 steel. The mean size of ferrite grain after tempering increases gradually along depth in the surface layer. However, the growth of ferrite grain occurs in the nanocrystalline specimen tempering at 1033 K due to the decrease of strain to a certain extent with increasing the depth from the top surface layer. The typical oxide scales are the double-layer as Fe2O3 and FeCr2O4 in T91 steel with nanocrystalline microstructure. The growth rate obviously decreases and the Cr content of inner oxide layer increases in T91 steel with nanocrystalline surface layer. T91 steel with nanocrystalline microstructure rapidly forms a FeCr2O4 inner oxide layer with higher Cr content that protects the material from further oxidation.
The authors are indebted to Dr. Zhenbo Wang of Shenyang National Laboratory for Materials Science for the cooperation in the SMAT experiments. Financial support from the National Natural Science Foundation of China (No. 51071090, No. 51201061), National Science and Technology ITER Major Project of the Ministry of Science and Technology of China (No. 2011GB108006) is acknowledged.