2015 Volume 55 Issue 1 Pages 312-318
2015 Volume 55 Issue 1 Pages 312-318
The range of chemical compositions that can obtain an austenitic single structure was defined for medium-manganese (Mn) carbon (C) steels. Among the potential compositions, Fe-5%Mn-4%Cr-(0.8–1.4)%C (mass%) was selected as the optimized composition range to form a stable austenitic structure. The tensile properties and deformation substructure were investigated in the austenitic steels having this composition. The work hardening behavior of the steels varied depending on the carbon content, which was closely related to the deformation microstructure. In the 0.8%C steel, both a deformation-induced martensitic phase as well as the formation of deformation twins generated a high work hardening until fracture. With an increasing carbon content, which increased the stacking fault energy (SFE), the deformation tended to shift towards dislocation slipping, resulting in a lower work hardening rate. This trend appears similar to conventional twinning-induced plasticity steel where the work hardening behavior is tied to the SFE.
High manganese steel with an austenitic single structure has a high strength with a superior resistance to erosion, making it popular as a high strength structural steel for engineering works, construction machinery, and rail crossings. Hadfield steel (Fe-13%Mn-1%C, mass%) is a typical high-Mn steel popular for its resistance to friction and impingement, and similar modified steels having a superior corrosion resistance and work hardening have also been developed.1,2,3) Research is ongoing for high-Mn steels to transfer their application to the automobile industry. Twinning-induced plasticity (TWIP) steel ((Fe-(15–30)%Mn-Al-Si)4,5) is a potential candidate as it can obtain high work hardening through the formation of deformation twins. However, these steels have not become widely used due to their low productivity and high alloy costs. In terms of saving natural resources, it is preferable to reduce the quantities of Mn used in steel production.
Based on these limitations, medium-Mn steel has become the focus as a next generation high strength structural steel. The medium-Mn steel consists of (3–7)%Mn and has an excellent balance of strength, elongation, and toughness through the transformation induced plasticity (TRIP) effect, which controls the austenite stability with optimized production processes.6,7,8,9) Thus the alloy element (Mn) quantity can be reduced compared to conventional high-Mn austenitic steel. Recently, research on 5%Mn steel has increased in Asian countries, and there is considerable work on improving the tensile properties9) and clarifying the phase transformation behavior.10)
However, when reducing the Mn content from high-Mn austenitic steel, the transformation to martensite occurs during cooling through an increased martensite start temperature (Ms temperature). Therefore, duplex structures such as (austenite + martensite) and (austenite + ferrite) are formed in medium-Mn steel. Indeed, recent studies have discussed how to best optimize the duplex structures to improve steel properties. If the austenitic single structure can be obtained in these medium-Mn alloys, then its favorable characteristics such as high work hardening, elongation, and non-magnetic properties can be applied for use in structural products. To achieve this degree of microstructural control, the alloy focus should be on C, with its considerable effect on austenite stability, while the other alloy components should be a secondary focus.
In this study, the range of chemical compositions that can obtain an austenitic single structure were defined for medium-Mn-C steels (5%Mn), and then their mechanical properties were investigated. As accessorial elements, Cr, Ni, and Mo were available to add to the steel for controlling the Ms temperature. Of these, Cr was selected to reduce the alloy cost, due to it being an abundant resource, and Fe-5%Mn-Cr-C austenitic steels were fabricated in this study.
Two types of ingots were used in this study for the varied investigations. Small ingots (30 g) were prepared using a vacuum induction melting furnace to investigate the optimum composition range that forms a stable austenitic structure. Table 1 shows the chemical compositions. The Mn, Cr, and C contents were varied to produce 12 kinds of steel. The small ingots were hot-rolled at 1373 K to a 6 mm thickness, reheated at 1373 K for 180 s (solution-treatment), followed by quenching in water. The hot-rolling reduction ratio was 2 mm/pass. To avoid a temperature decrease of the steel plate, a reheating was performed for each pass. In addition, larger 10 kg ingots were used to investigate the mechanical properties. The large ingot chemical compositions are shown in Table 2. These ingots were hot-rolled at 1473 K to a 15 mm thickness, with a 1 mm/pass reduction ratio. The hot-rolling temperature was kept constant using the same method as for the small ingots. The hot-rolled steel plates were solution-treated at 1473 K for 600 s, followed by quenching in water.
| C | Mn | Cr | |
|---|---|---|---|
| 5Mn-4Cr-0C~1.4C | 0, 0.2, 0.5, 0.8, 1.1, 1.4 | 5 | 4 |
| 5Mn-8Cr-0.5~0.7C | 0.5, 0.6, 0.7 | 5 | 8 |
| 5Mn-12Cr-0.3~0.5C | 0.3, 0.4, 0.5 | 5 | 12 |
| C | Si | Mn | P | S | Cr | |
|---|---|---|---|---|---|---|
| 0.5C steel | 0.50 | 0.22 | 4.88 | 0.007 | 0.0037 | 3.99 |
| 0.8C steel | 0.77 | 0.22 | 4.80 | 0.008 | 0.0037 | 3.86 |
| 1.1C steel | 1.10 | 0.22 | 4.78 | 0.008 | 0.0048 | 3.86 |
| 1.2C steel | 1.22 | 0.22 | 4.71 | 0.008 | 0.0031 | 3.75 |
The solution-treated sample microstructures were observed using an optical microscope. The specimen was wet polished and etched with Vilella’s solution (glycerin: hydrochloric acid: nitric acid = 2:2:1). To investigate the deformation substructure, the grain orientation was mapped using a field-emission scanning electron microscope with electron back scattering diffraction (FE-SEM/EBSD). Specimens 1 cubic millimeter in size were removed from close to the fracture position and subjected to a twin-jet method with a 10% perchloric acid-90% acetic acid solution. 300 μm×300 μm area was analyzed under the condition of step size: 0.3 μm and acceleration voltage: 20 kV. X-ray diffractometry with curved imaging plate area detector (RINT RAPID) was conducted to analyze the deformation substructure in the fractured specimens, with the specimens wet polished and etched using a phosphoric acid and chromic acid solution (H3PO4:CrO3 = 2:1). Co Kα radiation was used under the condition of voltage: 40 kV, current: 30 mA and radiation time: 360 s. The Ms temperature was measured using a thermodilatometer on 4 × 4 × 10 mm specimens. The cooling rate was 1.7 K/s across a measurement range of room temperature to 173 K. Tensile tests were performed on plate specimens (reduced area: 1 mm thick × 3 mm wide × 6 mm long) with a 0.2 mm/min crosshead speed (initial strain rate: 5.6 × 10–4 s–1). A nominal stress-strain curve was obtained based on the load and crosshead displacement.
In order to obtain an austenitic single structure steel at room temperature, the chemical composition must be optimized to produce a thermodynamically stable austenitic single structure at the solution-treatment temperature and to suppress the martensitic transformation during cooling. The martensitic transformation can be suppressed by lowering the Ms temperature to room temperature. Although the Ms temperature decreases with increasing Mn, Cr, and C, content, the addition of Cr and C promotes carbide formation, making it difficult to obtain the austenitic single structure at the solution-treated temperature. On the other hand, the addition of Mn is preferable in high C austenitic steels, as it does not significantly affect the carbide solubility. To fabricate the austenitic single structure in the medium-Mn range, the balance between Cr and C was optimized in this study for a minimum use of Mn.
The effects of Mn, Cr, and C on the solubility of carbide were calculated using the Thermo-Calc software as shown in Fig. 1, to clarify the maximum carbon content that could be used. The solubility of carbide (solid line) is the boundary between the (austenite + carbide) phase and the austenitic single phase, and the region below this line corresponds to the Mn–Cr composition where all of the added C should be in a solid solution. This figure reveals that the Mn content does not affect the carbide solubility; however, increasing the Cr content decreases carbide solubility, making it easier to precipitate.

Iso-carbon solubility diagram at 1373 K for Fe–Cr–Mn–C alloy.
For example, in the case of the Fe-5%Mn-10%Cr alloy, the maximum carbon content that can be added is approximately 0.6 mass%, and the final chemical composition should satisfy this requirement with a Mn–Cr balance to lower the Ms temperature to room temperature. In this study, a 5%Mn was selected and the balance between Cr and C was discussed. Figure 2 shows the equilibrium phase diagram of the Fe-5%Mn-4%Cr-C alloy at 1373 K. The broken line denotes the chemical composition where the Ms temperature is equal to room temperature (298 K). The Ms temperature was estimated with Eq. (1),11) which was established for low-alloy carbon bearing steels.
| (1) |

Phase diagram for Fe-5%Mn-Cr-C alloy at 1373 K.

Optical micrographs of medium manganese steels water-cooled after solution treatment at 1373 K for 180 s. (a) Fe-5%Mn-12%Cr-0.5%C alloy, (b) Fe-5%Mn-8%Cr-0.5%C alloy, (c) Fe-5%Mn-8%Cr-0.7%C alloy, (d) Fe-5%Mn-4%Cr-0.5%C alloy, (e) Fe-5%Mn-4%Cr-0.8%C alloy, (f) Fe-5%Mn-4%Cr-1.1%C alloy, (g) Fe-5%Mn-4%Cr-1.4%C alloy.

Structure diagram for Fe-5%Mn-Cr-C alloy at 1373 K.
From these results, the chemical composition range for obtaining an austenitic single structure was defined in medium-Mn steels by investigating various quantities of Mn, Cr, and C. In the case of higher Cr content, carbide can easily precipitate and it is difficult for the additional C to be in a solid solution. Additionally, the purpose of reducing elements within the alloy was not satisfied. On the other hand, in the case of lower Cr content, the amount of carbon required for the austenitic single structure is considerably higher, allowing carbide precipitation during cooling. In this study, a composition range of Fe-5%Mn-4%Cr-C was selected due to the carbide solubility and ability to reduce the elements in the alloy as much as possible.
3.2. Tensile Deformation Behavior and Deformation Substructure in the Fe-5%Mn-4%Cr-C AlloyTo produce steel plates for tensile testing, 10 kg ingots of the Fe-5%Mn-4%Cr-(0.5–1.2)%C (Table 2) were prepared, hot-rolled to size and underwent a solution treatment. It was confirmed that the solution-treated large ingot specimens exhibited similar microstructures to the small ingot specimens. The (martensite + austenite) structure was formed in the 0.5%C steel sample, and the austenitic single structure was obtained in the other specimens. In the 1.1%C and 1.2%C steels, a minimal amount of fine carbide was dispersed at the grain boundaries, due to the difference in the specimen size as shown in Fig. 5. As the specimen thickness was large compared to the samples in Fig. 3, the cooling rate decreased and carbide precipitates during the cooling. Figure 6 shows the X-ray diffraction patterns of these specimens, with the results corresponding to the optical micrographs. In the 0.5%C steel, the BCC diffraction peak as well as a face-centered cubic (FCC) peak (austenite) was detected, whereas only the austenite peak was detected for the (0.8–1.2)%C steels. The volume fraction of the BCC phase (martensite) was almost 30% for the 0.5%C steel. To estimate the Ms temperature, which relates to the austenite stability, a dilatation measurement was performed on the samples, with the results shown in Fig. 7. The Ms temperature of the 0.8%C steel was at 219 K, and it was below the measurement limit (173 K) for the 1.1%C and 1.2%C steels, indicating that the high carbon steels have a stabile austenite formation. Figure 8 shows the relationship between the measured hardness and the C content in these solution-treated specimens. The results of other austenitic steels with various quantities of C, Mn, Cr, and Ni are also indicated in this figure. The plot of the 0.5%C steel deviates considerably from the linear relationship of the austenitic steels (hatched band) due to the hard phase (martensite) formation. The results of the (0.8%–1.2%)C steels nearly align with the linear relationship obtained by previous researchers. Thus, the hardness of the C-bearing austenitic stable steels is predominately determined by the C content, while the effect of the other alloy elements is comparatively small.

Optical micrograph showing grain boundary carbide in Fe-5%Mn-4%Cr-1.1%C alloy.
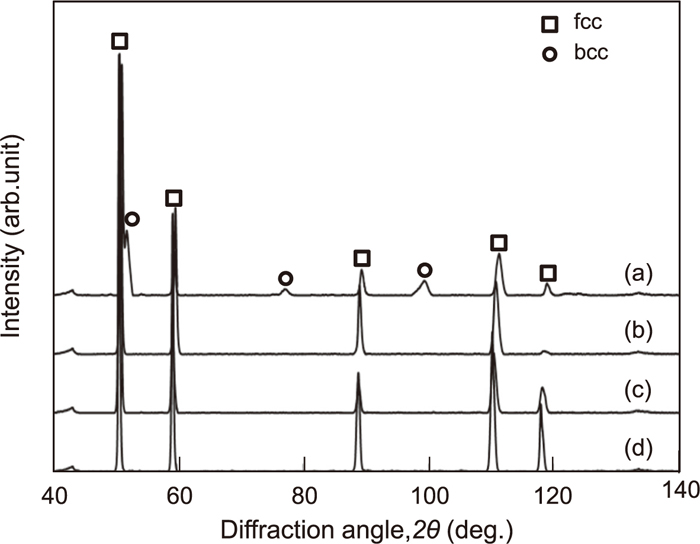
X-ray diffraction patterns of medium manganese 0.5%C (a), 0.8%C (b), 1.1%C (c) and 1.2%C (d) steels which were water-cooled after solution treatment at 1473 K for 180 s.

Dilatation during cooling in solution-treated medium manganese 0.8%C, 1.1%C and 1.2%C steels.

Relation between hardness and carbon content in solution-treated medium manganese steels. The data of other austenitic steels are also indicated for reference.
Tensile tests were conducted on these specimens with varied austenite stability and hardness values, with the results shown in Fig. 9. The yield stress increased with increasing C content and the austenite is strengthened by the C solid solution. After the maximum yield stress, a high work hardening was obtained with a clear increase in the flow stress. However, all of the steels in this study fractured at an early strain stage, with a lower elongation and tensile strength than seen for conventional austenitic steels. This appears to be the result of grain coarsening during the solution treatment and carbide precipitation at the grain boundaries. These problems will be discussed in detail henceforth. At the uniform deformation stage, serrations occur; it has been known that dynamic strain aging4,12,13) and deformation twins14) affect this phenomenon. To clarify the mechanism of such deformation behavior, the relationship between the work hardening characteristics and the deformation substructure was investigated. The true stress-strain curve and work hardening rate were plotted in Fig. 10. All samples fractured at the early strain stage prior to the plastic instability. The work hardening rate was around 1500 MPa after a 0.05 strain stage, and this is dependent on the C content. In the 1.2%C steel, the work hardening rate was higher at the early strain stage and then decreased gradually with deformation. This is similar to the dislocation slipping mechanism trend seen in steel with a high stacking fault energy (SFE).15) In comparison, the work hardening rate did not decrease at the later strain stage in the 0.8%C and 1.2%C steels; this was especially true for the 0.8%C steel, where the work hardening rate increased with deformation. These phenomena confirmed the deformation-induced martensite15) and deformation twin4) mechanism in the metastable austenitic steels. To investigate the C content effect on the deformation substructure, a microstructural analysis was performed on the samples fractured by the tensile tests. Figure 11 shows the optical micrographs of the fractured samples. The samples were cut parallel to the longitudinal sample direction. There are numerous substructure streaks in all samples, which appear to be the deformation-induced martensite and deformation twins. Figure 12 shows the X-ray diffraction patterns obtained for regions near the fracture (the distance from the fracture face is less than 100 μm). The BCC diffraction peak was detected in the 0.8%C steel, indicating that the deformation-induced martensite was formed by the tensile deformation. In comparison, there were no BCC peaks in the 1.1%C and 1.2%C steels, illustrating that these steels have stable austenite and the austenitic single structure was maintained after deformation.
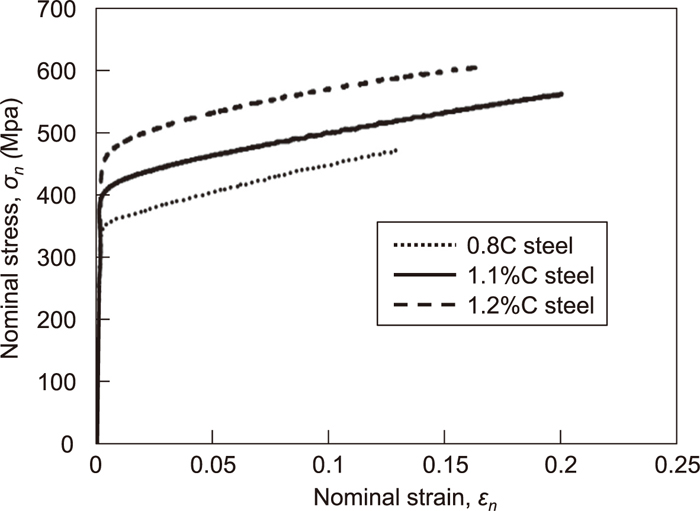
Nominal stress-strain curves of medium manganese 0.8%C, 1.1%C and 1.2%C steels.

Changes in true stress and work hardening rate as a function of true strain in medium manganese 0.8%C, 1.1%C and 1.2%C steels.

Optical micrographs observed near the fracture surfaces in tensile-tested medium manganese 0.8%C (a), 1.1%C (b) and 1.2%C (c) steels.

X-ray diffraction patterns obtained near the fracture surfaces of tensile-tested medium manganese 0.8%C (a), 1.1%C (b) and 1.2%C (c) steels.
Figure 13 shows the crystallographic orientation image maps, with (a) as the FCC and BCC analyses and (b) as a selected BCC map in the 0.8%C steel. Based on these results, it is apparent that the streak substructure does not correspond to the BCC phase (the deformation-induced martensite) but rather to a deformation substructure in the matrix. The deformation-induced martensite corresponds to a randomly distributed plate-like substructure. The crystallographic orientation relationship between the matrix and the deformation-induced martensite satisfied the Kurdjumov-Sachs (K-S) relationship. In the 1.1%C and 1.2%C steels, the BCC phase was not detected. As shown in Fig. 14, the streak substructure was deformation twins, where the twin plane is {111} and the common axis of rotation is <110>. The frequency of deformation twin formation was tended to be decreased with increasing C content.

Crystallographic orientation imaging maps of tensile-tested medium manganese 0.8%C (a) and (b) (bcc phase), 1.1%C (c) and 1.2%C (d) steels (parallel to tensile axis).

Pole figures showing crystallographic orientation relationship between austenite matrix and deformation twin.
From these results, the work hardening mechanism can be summarized for the different samples. In the 0.8%C steel, both the deformation-induced martensite and the deformation twins affected the high work hardening rate. In the 1.1%C steel, the deformation twin formation dominated the deformation substructure. However, in the 1.2%C steel, these deformation substructures are hardly formed, so dislocation slipping is the primary mechanism. Therefore, the work hardening rate decreased gradually with deformation. Further investigation is needed on the dynamic strain aging effect. In this study, the dynamic strain aging contribution is nearly identical among the three samples, because the Mn and Cr content, which interact with the C atoms, is equal.
3.3. Work Hardening Characteristics of 5%Mn-4%Cr-C SteelsIt is known that in general, the work hardening of austenitic steels depends on the SFE. Remy and Pineau16) indicated that the deformation substructure changes from a dislocation cell to ε martensite with decreasing SFE, leading to a work hardening rate increase. As for the Mn effect, it has been reported that the addition of 15% Mn resulted in a monotonously decreasing SFE.17) If the relationship between the Mn content and the SFE could be extrapolated to a lower Mn range, the 5%Mn steel could have a high SFE which is more than 30 mJ/m2. The effect of Cr on the SFE is smaller compared to other elements; Cr increases the SFE in some alloys, but decreases it in others.18) On the other hand, C has a strong effect on the SFE. If the equation suggested by Schramm and Reed19) could be applied, the addition of 1% C could increase the SFE to 410 mJ/m2. Considering these results, the Fe-5%Mn-4%Cr-C alloys in this study should have a high SFE and smaller work hardening than other austenitic steels. However, it has been noted that C promotes the formation of deformation twins or strengthens them,20) increasing the work hardening rate. It is also possible that the dynamic strain aging by the Mn–C clusters affects the work hardening behavior;12) however, thus far the exact mechanism has not yet been clarified.
The difference in the work hardening characteristics between the medium-Mn-C steels in this study and other austenitic steels were discussed in terms of their SFE. Olson and Cohen21) suggest using Eq. (2) to estimate the SFE as a function of the Gibbs free energy difference between the FCC phase and the hexagonal close-packed (HCP) phase (ΔGfcc→hcp).
| (2) |

Relation between carbon content and Gibbs free energy change by fcc to hcp transformation (293 K).

Relation between Gibbs free energy change by fcc to hcp transformation (293 K) and work hardening rate at 5% and 10% tensile deformations.
(1) The optimum chemical composition range for obtaining an austenitic single structure was defined for Fe-5%Mn-Cr-C alloys. In the case of a 4%Cr alloy, the addition of (0.8–1.4)% carbon enables the fabrication of medium-Mn austenitic steels.
(2) The work hardening behavior depended on the carbon content in the Fe-5%Mn-4%Cr-(0.8–1.2)%C (mass%) alloys. The 0.8%C steels had a high work hardening rate at both the early and later strain stages due to deformation-induced martensite and deformation twin formation. The 1.1%C also exhibited a high work hardening tendency due to deformation twins. However, in the case of the 1.2% steel, the austenite is more stable, suppressing deformation twins and the main mechanism is dislocation slipping, leading to a decreased work hardening rate.
(3) The work hardening rate of the Fe-5%Mn-4%Cr-(0.8–1.2)%C alloys is slightly lower than that of previously examined Fe–Mn–C TWIP steel. This appears to lower the frequency of deformation twin formation by the incremental stacking fault energy increase through the addition of carbon.