1. Introduction
Tundish was originally designed to be reservoir and distributor for liquid steel before continuous casting.1) The cleanliness of liquid steel has impact on quality of steel and has received many attentions in recent year. The role of tundish in inclusion removal has been focused by many researchers. The flow of liquid steel was optimized to prolong the residence time for steel in tundish in order to promote the removal of inclusion.2) Therefore, functions of tundish have been extended from original “reservoir and distributor” to control of steel temperature, chemistry and cleanliness.
Traditionally, tundish covering slag was designed to keep thermal insulation and protect reoxidation of steel. Many recent efforts have been made to improve capability of tundish covering slag to absorb non-metal inclusion.3) As the last step for inclusion removal in tundish, the dissolution of inclusion, e.g. Al2O3, in slag is of vital importance to increase the cleanliness of steel.
The dissolution of alumina in slag has been investigated by rotating finger, Confocal scanning laser microscopy, etc.4,5,6,7,8,9,10,11,12,13,14) The dissolution of Al2O3 rod in CaO–Al2O3–SiO2 -based slag under forced convection has been studied by many researchers using rotating finger method.4,5,6,7,8,9) It was found that diffusion in boundary layer was rate controlling and the dissolution rate increased with increasing rotating speed and temperature. Confocal scanning laser microscopy (CSLM) was employed to investigate the dissolution of Al2O3 particle in molten slag under natural convection11,12,13) and it was reported that Al2O3 dissolution process in CaO–Al2O3–SiO2 and CaO–Al2O3–MgO–SiO2 is controlled by boundary layer diffusion inside the slag phase. The Al2O3 dissolutions in many slags were found to be indirect and intermediate solid phases were found to form at the interface, which could bring strong influences on the overall dissolution.7,8,9,12,13)
It was reported that addition of alkaline oxide could decrease the viscosity of slag.15) Considering that diffusion in boundary layer could be enhanced by decrease of viscosity, alkaline oxides have a potential to increase the dissolution rate of inclusion in slag. However, Yu et al.16) investigated effect of alkaline oxide in tundish slag on cleanliness of steel and found Na2O and Li2O has minimal effect on cleanliness of steel while K2O could increase the cleanliness of steel. However, to the best knowledge of the present authors, there is no report on dissolution of Al2O3 in CaO–Al2O3–MgO–SiO2–Na2O system in literature. To elucidate the influence of Na2O on inclusion absorption of tundish slag, the dissolution of Al2O3 rod in CaO–Al2O3–MgO–SiO2–Na2O slag at 1823 K was investigated by employing rotating finger method in this work. The dissolution mechanism was elucidated by investigation with varied rotating speed and Na2O content in slag.
3. Results and Discussion
3.1. Effect of Rotation Speed on Dissolution Rate of Al2O3
Figure 1 showed decreases in radius of rod in slag with Na2O%=2.4% as functions of dissolution time at different rotation speeds. Three rotation speeds of 100, 150 and 200 rpm were employed to elucidate the underlying mechanism for dissolution. It could be seen from the figure that decrease in radius of rod increases linearly with increase of dissolution time at the same rotation speed, and decrease in radius increases with increase of rotation speed at the same dissolution time. The decreases in radius of rod at static condition (with no rotation) were also shown in Fig. 1. It was found that the decrease of radius of rod in static condition is much lower than that in rotating condition, indicating that the dissolution was significantly enhanced by forced convection induced by rod rotation.
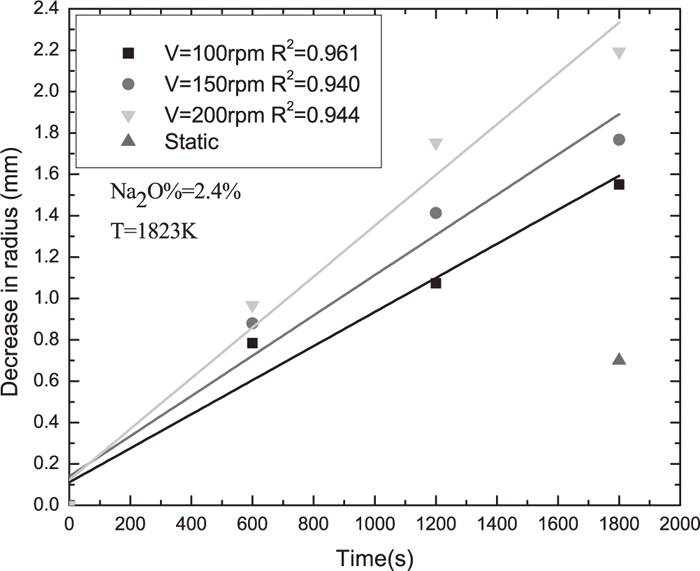
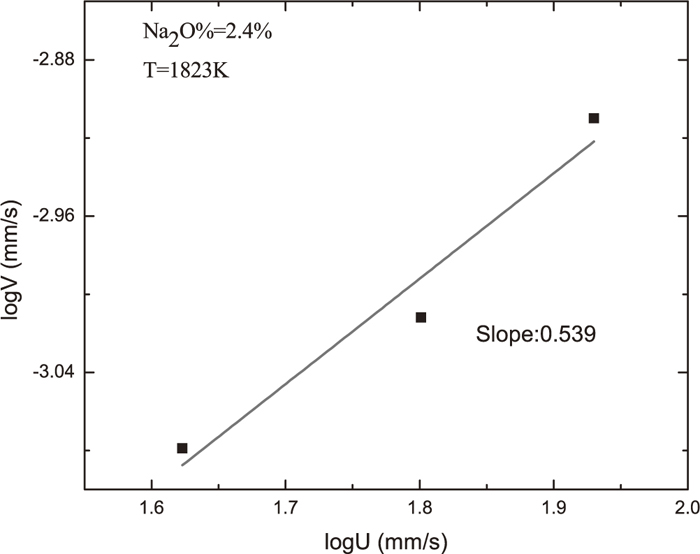
The rate of dissolution could be calculated by fitting decrease in radius with respect to dissolution time. Considering the possible presence of the Taylor’s vortex, decrease in radius data in Fig. 1 was fitted with lines with intercepts. Data in Fig. 1 could be fitted well using line with intercepts and the correlation factors R2 were shown in Fig. 1. It could be seen from Fig. 1 that dissolution rate as shown by slope of fitting line increases with increase of rotation speed. Assuming that mass transfer of Al2O3 in molten slag is controlling step for dissolution of Al2O3 in slag, a relationship between the dissolution rate and the periphery velocity could be established as follows:4)
where b represents a constant and U represent the periphery velocity U (cm/s) which could be calculated as
U=
πdm/60, where
d represents the mean diameter of cylinder in mm and m represents number of revolutions per min); Exponent
s has been reported
4,5,6) in the range of 0.50 to 0.80. The relationship between lnV and lnU was shown in
Fig. 2. It can be seen that the lnV is linearly proportional to lnU, which indicates that the dissolution of alumina into flux is controlled by mass transfer in the flux phase. The calculated value of s as the slopes of the lines is about 0.53 and falls within the range of 0.50 to 0.80 reported before. Thus, it is presumed that the rate-controlling step for the dissolution of alumina is mass transfer within the boundary layer in the present slag. Sridhar
et al.11) investigate the dissolution of Al
2O
3 particle in 19.5%Al
2O
3-33.4%CaO-7.3%MgO-39.5%SiO
2 (in mass percentage) slag by employing CSLM technique under natural convection. They found that the dissolution rate from 50–80 mm down to 10 mm could be described by considering boundary layer diffusion as dissolution. The dissolution mechanism proposed in the present work is consistent with theirs in spite of the fact that the present dissolution took place under forced convection.
3.2. Effect of Na2O Addition on Dissolution of Al2O3
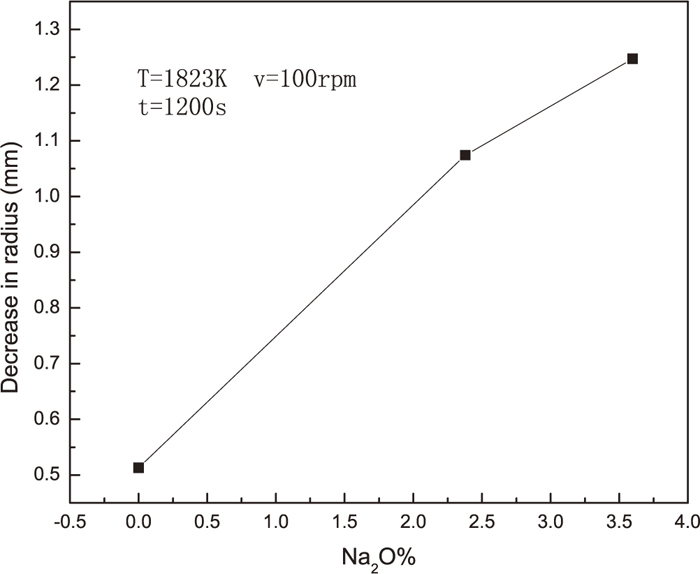
Figure 3 showed that decreases in radius as functions of Na2O content in slag with Al2O3 dissolving for 1200 s at 100 rpm. It could be seen that decrease in radius increases with increase of Na2O content in slags, which indicates that addition of Na2O would promote the dissolution of Al2O3 in the present slag. The present results indicate that the addition of Na2O would be beneficial to dissolution of alumina in CaO–Al2O3–MgO–SiO2 system, and thereby help to removal of inclusion in tundish.
It has been speculated that the dissolution of alumina in the present slag is determined by the mass transfer in the molten slag. Mass transfer flux during the dissolution can be represented by the following equation:
|
J=k(
C
sA
l
2
O
3
-
C
nA
l
2
O
3
)
| (2) |
where k is the mass transfer coefficient (cm/s),
CbAl2O3 and
CsAl2O3 are the concentration of Al
2O
3 in the bulk slag and at the interface between solid and liquid respectively,
CsAl2O3−
CbAl2O3 value represents the thermodynamic driving force for dissolution.
Mass balance of Al2O3 gives,
|
ρ
A
l
2
O
3
⋅A(-dr/dt)=A⋅
J
A
l
2
O
3
| (3) |
where A is the surface area between solid and liquid (cm
2) and
ρAl2O3 is the mass density of solid (g/cm
3). Combining
Eqs. (3) and
(4), one can get
|
V=-dr/dt=k(
ρ
flux
100
ρ
A
l
2
O
3
)
Δ%A
l
2
O
3
| (4) |
where Δ(%Al
2O
3)=(%Al
2O
3)
s−(%Al
2O
3)
b represents the chemical driving force for dissolution, (%Al
2O
3)
b and (%Al
2O
3)
s are mass percentage of Al
2O
3 in the bulk slag and at the solid-liquid interface respectively. (%Al
2O
3)
s could be obtained in term of liquidus boundary in phase diagram.
From Eq. (4), the dissolution rate was mainly determined by thermodynamic driving force and mass transfer coefficient. Dissolution rates of Al2O3 are accordingly enhanced. (%Al2O3)s value represents the saturated Al2O3 content in slag which could be obtained from phase diagram. Due to its low melting point (1132°C), Na2O has the ability to decrease the melting temperature of slag. It could be shown in phase diagram of CaO–Al2O3–SiO2–Na2O18) that addition of Na2O would decrease liquidus temperature of slags and enlarge the liquidus area in phase diagram, thereby increasing saturated Al2O3 content and thermodynamic driving force for dissolution increases. Accordingly, the dissolution rates of Al2O3 in slag increases.
On the other hand, the mass transfer coefficient k in the slag phase is presented by levich equation:19)
|
k=0.62
D
2/3
ν
-1/6
ω
1/2
| (5) |
where
D is the diffusion coefficient of components in slag,
ν is the kinematic viscosity of liquid and
ω is the angular velocity of rod. As can be seen in
Eq. (5), k is proportional to D
2/3. The experimental data for diffusion coefficient of components in slag is very few. However, diffusion coefficient could be related to viscosity of slag by Stokes-Einstein equation:
where
kB is Boltzmann’s constant, r is radius of atoms or ions,
η is viscosity of slag. Therefore, the effect of Na
2O on viscosity of slag could be employed to interpret the variation of dissolution rates.
Na2O was usually regarded as a network modifying oxide in silicate network.20) It can broke complex structural units into simple units, and thereby decrease the viscosity of slags. The effect of Na2O on viscosity of slags was investigated by many authors. Kim et al.15) measured viscosity of CaO–SiO2–MgO–Al2O3–Na2O slag with Na2O%=0−10%, and found that the slag viscosity at 1773 K decreased with increasing Na2O contents. One of the present authors presented a model to calculate viscosity of multi-component slag.21,22) The model has been applied to calculate viscosity of many multi-component slag e.g. CaO–SiO2–MgO–Al2O3, CaO–SiO2–Al2O3–Na2O systems and a good agreement between calculated and measured viscosity values has been achieved with mean deviation less than 25%. Therefore, the model was employed to calculate viscosity of the present slags. Calculation results for viscosity of the present slags were shown in Fig. 4. It could be seen the figure that viscosity of the present slag is lowered by increase of Na2O content, which is in consistent with viscosity measurements by Kim et al. According to Stokes-Einstein equation, diffusion coefficient of components in slags is inversely proportional to viscosity of slags. The increase of Na2O content in slag decreases the viscosity of slags, thereby increase the diffusion coefficient of components in slags. According to Eq., the increasing of diffusion coefficient of components in slag would lead to acceleration of dissolution of Al2O3 in slag.
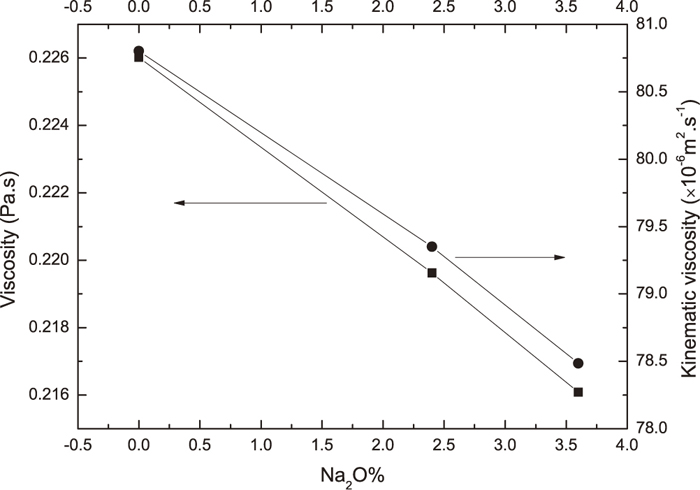
It could be also seen from Eq. (5) that k is inversely proportional to ν1/6. kinematic viscosity could be calculated from viscosity and density of slag as η/ρ. Densities of slags investigated in the present work was calculated by using the equation proposed by Mills et al.23) The calculated kinematic viscosity values are also shown in Fig. 4. It could be seen from the figure that kinematic viscosity of slag decreases with increase of Na2O content. Therefore, according to Eq. (6), decrease of kinematic viscosity of slag with increase of Na2O content in slag would also help to increase of mass transfer coefficient, and thereby increase of Al2O3 dissolution rate in slag.
3.3. Interface between Al2O3 and Slag
Figure 5 showed micrographs of Al2O3-slag interface for slags with different Na2O content. The alumina rod was rotated in molten slag at the speed of 100 rpm for 1200 s. The Dark grey part in micrographs is Al2O3 rod, and the light grey part is slag. It could be seen that there are many dark grey crystals in slag phases. Both equiaxed and columnar crystals could be found in all slags. EDS showed the composition of equiaxed crystals and columnar crystals are MgAl2O4 and CaAl4O7 respectively. The composition analyses along lines across Al2O3-slag interface were also performed for all samples and shown in Fig. 5. Composition gradients could be found in all samples. There are also many composition fluctuations in slag phase due to precipitation of many crystals.
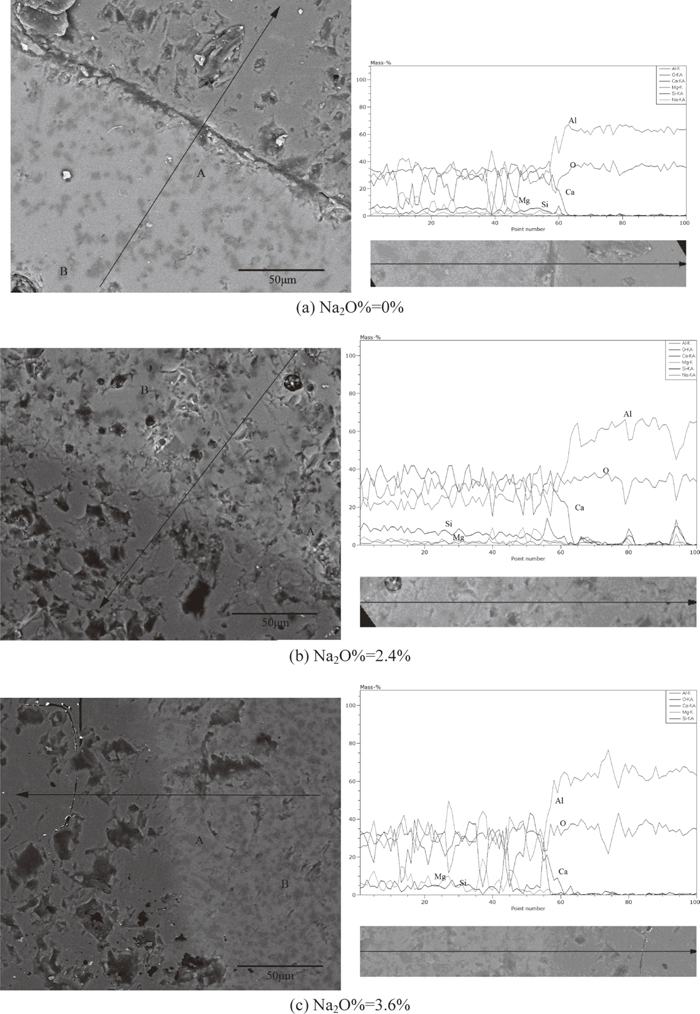
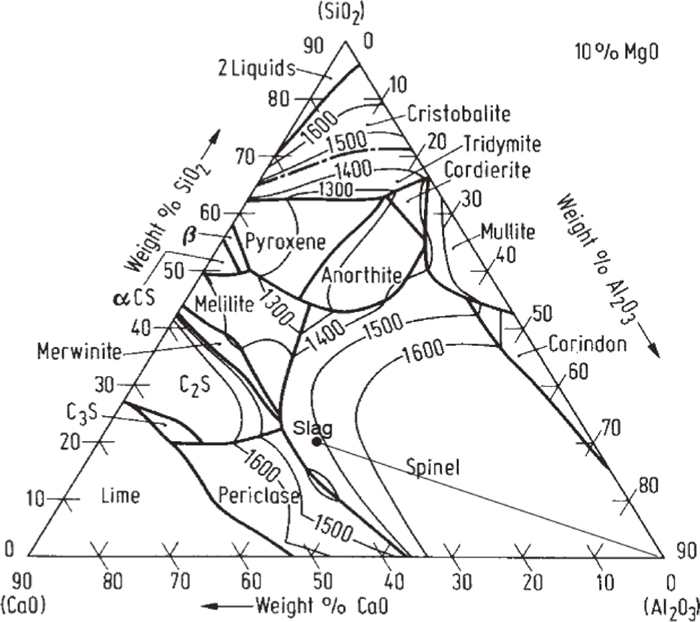
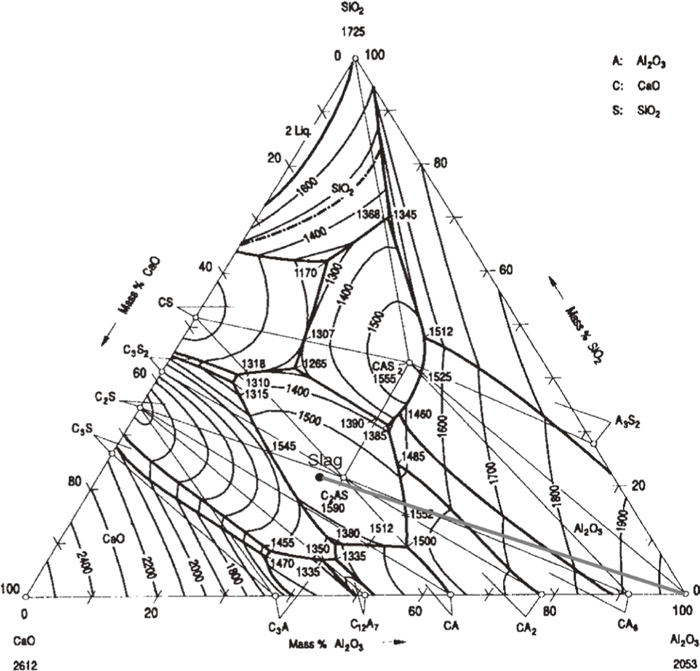
The melting points of MgAl2O4 and CaAl4O7 are higher than temperature in the present experiments. Therefore, MgAl2O4 and CaAl4O7 should be in the solid state at experimental temperature and formed by dissolution of Al2O3. The formation of many intermediate phases indicates that the dissolution of Al2O3 in the present slag is not in a direct manner but proceeds indirectly. The indirect dissolution of Al2O3 includes two steps as follows. Firstly, the dissolution of Al2O3 in slag leads to the formation of intermediate solid phases at or near Al2O3-slag interface. Then, intermediate phases gradually dissolve into slag. The formation of intermediate phases as MgAl2O4 and CaAl4O7 could be interpreted with the aid of phase diagrams of CaO–Al2O3–SiO2–(MgO).18) Figure 6 showed the phase diagram of CaO-Al2O3-SiO2-10%MgO. The composition of the present slag was marked on the phase diagram. As Al2O3 gradually diffused into slag, the composition would change along with the line slag-(Al2O3) in Fig. 6. It could be seen from Fig. 6 that the liquidus temperature of slag increases with increase of Al2O3 content. The line slag-(Al2O3) is apparently located in the primary crystal field of MgAl2O4. Therefore, the enriched Al2O3 content in slag would lead to the precipitation of MgAl2O4. It could be seen from Fig. 5 that MgAl2O4 precipitated in all samples. Sandhage et al.7,8,9) studied the dissolution of Al2O3 in CaO–Al2O3–MgO–SiO2 slags, and observed the formation of MgAl2O4 during dissolution. Valdez et al.12) Investigate the dissolution of Al2O3 in CaO–Al2O3–MgO–SiO2 slags using CSLM technique. They also found the formation of MgAl2O4 as an intermediate solid phase. The precipitation of MgAl2O4 would gradually lower the concentration of MgO in slag. Therefore, phase diagram of CaO–Al2O3–SiO2 system18) is more appropriate to interpret the precipitation of secondary crystal. Figure 7 showed the phase diagram of CaO–Al2O3–SiO2 system. The composition the present slag (The MgO and Na2O contents were neglected) was marked on the diagram. The dissolution of Al2O3 in slag would lead to change of composition which could be represented by line slag- Al2O3 in Fig. 7. As shown in the figure, the liquidus temperature of slag increases as Al2O3 concentration increases. The gradual increase of Al2O3 content in slag would cause that composition of slag successively pass through the primary crystal fields of Ca2Al2SiO7, CaAl4O7, CaAl12O19 and Al2O3. Therefore, CaAl4O7 and CaAl12O19 could precipitate from liquid and become possible intermediate solid phases during dissolution of Al2O3. Due to possible kinetic factors, only formation of CaAl4O7 could be observed in the present work. Park et al.13) investigated the dissolution of Al2O3 in CaO–Al2O3–SiO2 slag using CSLM technique, and found reaction products such as Ca2Al2SiO7, CaAl4O7 and CaAl12O19 formed on the surface of Al2O3 particle. The present author reported17) the kinetics of Al2O3 in CaO–Al2O3–CaF2 flux and found the existence of CaAl4O7 as an intermediate solid phase during Al2O3 dissolution. These previous reports are in line with the present work with regard to intermediate solid phase during dissolution.It could be also found in micrographs that CaAl4O7 particles are closer to Al2O3 rod than MgAl2O4. Due to its small size and equiaxed morphology, MgAl2O4 is easier to be thrown out by the centrifugal force induced by rotation. Therefore, the MgAl2O4 layer is located at a distance from rod.
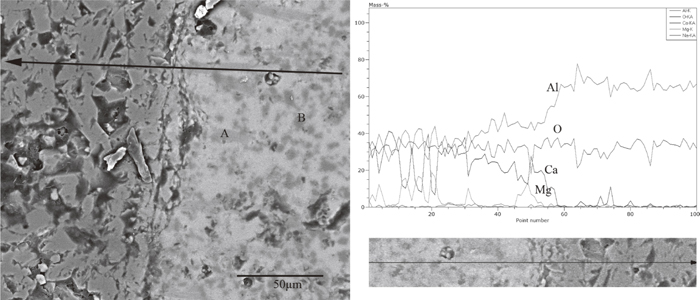
It would be interesting to investigate the interface between rod and slag under static condition. Figure 8 showed the micrograph and the line composition analysis of interface between rod and slag with Na2O%=2.4% under static condition. It could be seen from the figure that there are many equiaxed and columnar particles existing in slag phase. The equiaxed and columnar particles are MgAl2O4 and CaAl4O7 respectively. This indicates that the intermediate phases are not changed with introduce of rotation for slag with Na2O%=2.4%. However, comparing Fig. 8 with Fig. 5(b), it could be found that the sizes and numbers of intermediate phases in slags under static condition are larger than that in slags under forced convection. It has been shown that forced convection by rotating rod accelerates the dissolution of Al2O3. Accordingly, force convection should be beneficial to overall dissolution by promoting the dissolution of intermediate solid phases (the second stage). The composition analysis was carried out along a line across slag-Al2O3 interface. Compared with samples in forced convection, a mild composition gradient was found in samples under static condition, which is due to the weaker mass transfer under static condition.
According to above discussion, the dissolution mechanism for Al2O3 could be summarized as bellows. At the first stage, Al2O3 was gradually transferred into slag, and the supersaturation of Al2O3 would lead to precipitation of the first intermediate solid phase MgAl2O4. The second intermediate solid phase CaAl4O7 could precipitate from slag with further supersaturation of Al2O3. At the second stage, intermediate solid phases gradually dissolved into slag. The steel flow induced by rod rotation would be beneficial to the dissolution of these intermediate solid phases.
It could be inferred from the present work that the dissolution of Al2O3 in basic tundish slag could be enhanced with addition of Na2O. However, the addition of Na2O leads to more intermediate solid particles which could be obstacles for complete dissolution of Al2O3. Yu et al.16) reported minimal effect of Na2O in tundish slag on cleanliness of steel. Since removal of inclusion include also separation of inclusion at slag-metal interface other than dissolution of inclusion into slag, the elucidation of effect of Na2O addition on the cleanliness of steel still needs further studies in the future.