2015 Volume 55 Issue 11 Pages 2310-2317
2015 Volume 55 Issue 11 Pages 2310-2317
Phase equilibria and liquidus temperatures in the CaO–SiO2–Al2O3–MgO system with CaO/SiO2 weight ratio of 1.30 have been experimentally determined by means of high temperature equilibration and quenching technique followed by electron probe X-ray microanalysis. Isotherms between 1723 and 1773 K were determined in the primary phase fields of melilite, dicalcium silicate, spinel, merwinite and periclase that are relevant to ironmaking slags. Effects of Al2O3, MgO and CaO/SiO2 ratio on the liquidus temperatures have been discussed. Compositions of the solid phases corresponding to the liquidus have been accurately measured that will be used for development of the thermodynamic database.
The blast furnace (BF) process continues to be the principal technique used for ironmaking across the world. Recently, with the increasing trend of utilization of low grade iron ores, and injection of coal, the BF operation confronts the new challenge of low gas permeability and formation of hearth accretion.1,2,3) To ease this issue, accurate data on the phase diagram of the BF slag are required. The oxide system CaO–SiO2–Al2O3–MgO forms a base for ironmaking slags. A series of pseudo-ternary phase diagrams sections in the system CaO–SiO2–Al2O3–MgO have been summarised in Slag Atlas4) at fixed concentrations of Al2O3 or MgO that are mainly based on the works reported by Osborn et al.,5) Gutt and Russel6) and Cavalier and Sandrea-Deudon.7) However, the isotherms reported were in 100 degrees interval and there are significant gaps in the composition range related to the ironmaking slags. In addition, the pseudo-ternary sections reported as CaO–MgO–SiO2 at fixed Al2O3 or Al2O3–CaO–SiO2 at fixed MgO cannot accurately predict the effect of MgO/Al2O3 on liquidus temperature which is important for the current BF operations. It has also been demonstrated in recent studies that significant differences are observed for the phase diagrams in the Al2O3–CaO–MgO–SiO2 system between the new results8,9,10,11,12) and those reported in the early research.5,6,7) On the other hand, solid solutions presented in the system Al2O3–CaO–MgO–SiO2, in particular melilite phase in which industrial slags are operated, are important for optimisation of the thermodynamic database. These compositions of solid solutions could not be accurately measured by previous techniques. Hence, accurate phase equilibria data are of importance for both industrial and scientific interests.
The BF slags can be classified to be primary slags, bosh slags and final slags during descent in the BF. Most of the final BF slags have the weight ratio of CaO/SiO2 between 1.10 and 1.30.13,14,15) The bosh slag in the upstream of the hearth generally has a higher CaO/SiO2 ratio than the final slag. In the current work, the liquidus surfaces in the CaO–SiO2–Al2O3–MgO system with CaO/SiO2 ratio of 1.30 have been investigated that are relevant to BF bosh and final slags.
The selection of an appropriate pseudo-ternary section in a multi-component system is important for efficient research and for easy use of the experimental information in industrial practice. In the present study, the phase diagram is presented in the form of pseudo-ternary section MgO–Al2O3–(CaO+SiO2) with CaO/SiO2 of 1.30, as shown in Fig. 1. There are a couple of advantages for choosing MgO, Al2O3 and (CaO+SiO2) as the end members of the pseudo-ternary section.

The pseudo-ternary section with CaO/SiO2 = 1.30 in the composition tetrahedron CaO–SiO2–MgO–Al2O3.
(a) With increasing usage of low grade iron ore, effects of Al2O3 and MgO on liquidus temperatures are important and can be examined accurately.
(b) CaO/SiO2 ratio (binary basicity) is commonly used by process operators to control slag composition. The CaO/SiO2 ratio does not change significantly in a particular plant. The presentation of the experimental results in the form of this pseudo-ternary section means that the information can be readily used by plant engineers to better understand and control the process.
(c) Ternary basicity (CaO+MgO)/SiO2 is important to evaluate sulphur capacity and viscosity of the slag. The pseudo-ternary phase diagram MgO–Al2O3–(CaO+SiO2) enables the liquidus temperatures to be analysed as a function of (CaO+MgO)/SiO2 ratio at fixed Al2O3 concentrations.
2.2. Experimental ProcedureThe experimental method used in the present study involves high temperature equilibration, quenching and electron probe X-ray microanalysis (EPMA).16,17) High-purity reagent powders of Al2O3, SiO2, MgO, CaCO3 were used as starting materials and thoroughly mixed in an agate mortar after weighing. The mixtures were pelletized prior to equilibration, and approximately 0.2 g samples were placed in graphite crucibles at predetermined temperatures in a high-purity Ar atmosphere. The experiments were carried out using a vertical electric resistance furnace. The sample was equilibrated for times from 8 to 24 hours depending on the composition and temperature. A Pt-30 pct Rh/Pt-6 pct Rh thermocouple placed in an alumina sheath was located adjacent to the sample to monitor the temperature. The thermocouple was calibrated periodically using a reference thermocouple supplied by National Measurement Laboratory (CSIRO, Melbourne, Australia). The temperature of the furnace was controlled within 2 K and overall temperature uncertainty is within 5 K.
The sample inside a graphite crucible was first attached to a Mo wire and placed in the cool zone of the furnace. The bottom end of the recrystallised alumina reaction tube was sealed with a glass window. After ultrahigh purity Ar (total impurities ≤ 5 ppm, oxygen ≤ 1 ppm) was passed through the furnace tube for 30 minutes to remove the air, the sample was raised into the hot zone to a position adjacent to the thermocouple. The experiment was usually carried out in two steps. The first step was to premelt the sample at a temperature higher than the anticipated liquidus to ensure that equilibrium was approached through precipitation from the melt. The sample was then equilibrated at the desired temperature for a time sufficient to achieve equilibrium.
2.3. Sample ExaminationThe samples were directly quenched into water after the equilibration, then dried, mounted and polished for metallographic analysis. The rapid quenching of these silicate slags to room temperature converted the liquid phase to homogenous glass. The crystalline solids present at temperature were also retained on quenching without change in their shapes and compositions. The microstructures were examined by scanning electron microscopy coupled with energy-dispersive spectroscopy analysis (SEM-EDS). Compositions of the liquid and solid phases were measured by a JEOL JXA-8200 Electron Probe X-Ray Microanalyser (EPMA) with Wavelength Dispersive Spectrometers (WDS). An accelerating voltage of 15 kV and a probe current of 15 nA were used. The Duncumb-Philibert ZAF correction procedure supplied with JXA-8200 was applied. The standards used for EPMA from Charles M. Taylor Co. (Standard, CA) include alumina (Al2O3) for Al, magnesia (MgO) for Mg, wollastonite (CaSiO3) for Ca and Si. The average accuracy of the EPMA measurements is within 1 wt%.
Over 150 experiments have been carried out in the CaO–SiO2–Al2O3–MgO system with the CaO/SiO2 weight ratio of 1.30. Dicalcium silicate, spinel, merwinite, periclase and melilite were found to be the primary phases in the composition range investigated. In the primary phase fields of dicalcium silicate, merwinite and melilite, CaO/SiO2 ratio in the liquid was usually lower than 1.30 as CaO/SiO2 ratio in these solid phases are higher than 1.30. The difficulty in controlling CaO/SiO2 ratio at 1.30 can be solved in preparing initial slags with slightly higher CaO/SiO2 ratios. The compositions of liquid and solids measured by EPMA covering Al2O3 concentration up to 35 wt%, MgO up to 20 wt%, are presented in Table 1 and plotted in Fig. 2. The liquidus with CaO/SiO2 ratio much lower than 1.30 were not used in constructing phase diagram. Typical microstructures observed in the quenched samples are presented in Fig. 3. Figure 3(a) shows the equilibrium of liquid with melilite at temperature. Figures 3(b) and 3(c) show the equilibrium of liquid with dicalcium silicate and liquid with spinel respectively. Figure 3(d) shows the equilibrium of liquid with merwinite and dicalcium silicate. EPMA measurements show that the compositions of dicalcium silicate (Ca2SiO4), spinel (MgO·Al2O3), merwinite (3CaO·MgO·2SiO2) and periclase (MgO) are close to their stoichiometry. Melilite is the solid solution between akermanite (2CaO·MgO·2SiO2) and gehlenite (2CaO·Al2O3·SiO2).
| Experiment No. | Temperature (K) | Phases | Composition (wt%) | CaO/ SiO2 | |||
|---|---|---|---|---|---|---|---|
| CaO | MgO | Al2O3 | SiO2 | ||||
| Liquid | |||||||
| 33 | 1693 | Liquid | 48.6 | 0.0 | 13.0 | 38.3 | 1.27 |
| 31 | 1703 | Liquid | 46.0 | 5.4 | 11.9 | 36.7 | 1.25 |
| 118 | 1703 | Liquid | 40.1 | 12.0 | 18.0 | 29.9 | 1.34 |
| 30 | 1723 | Liquid | 40.4 | 10.1 | 17.3 | 32.1 | 1.26 |
| 10 | 1723 | Liquid | 46.1 | 5.4 | 12.0 | 36.5 | 1.26 |
| 14 | 1723 | Liquid | 48.6 | 0.0 | 13.1 | 38.3 | 1.27 |
| 29 | 1723 | Liquid | 48.1 | 0.0 | 14.6 | 37.3 | 1.29 |
| 12 | 1723 | Liquid | 47.6 | 2.0 | 13.8 | 36.6 | 1.30 |
| 53 | 1723 | Liquid | 40.1 | 10.8 | 18.5 | 30.6 | 1.31 |
| 121 | 1723 | Liquid | 45.5 | 5.6 | 14.2 | 34.7 | 1.31 |
| 109 | 1723 | Liquid | 39.3 | 10.4 | 20.5 | 29.8 | 1.32 |
| 104 | 1723 | Liquid | 48.4 | 0.0 | 14.9 | 36.6 | 1.32 |
| 40 | 1723 | Liquid | 40.7 | 11.9 | 17.4 | 30.0 | 1.36 |
| 35 | 1733 | Liquid | 44.4 | 5.3 | 14.8 | 35.5 | 1.25 |
| 36 | 1743 | Liquid | 45.7 | 0.0 | 18.3 | 35.9 | 1.27 |
| 26 | 1753 | Liquid | 49.8 | 0.0 | 11.1 | 39.1 | 1.27 |
| 37 | 1753 | Liquid | 38.6 | 10.1 | 21.2 | 30.1 | 1.28 |
| 159 | 1753 | Liquid | 46.7 | 1.9 | 17.4 | 34.0 | 1.37 |
| 160 | 1753 | Liquid | 45.7 | 3.7 | 17.4 | 33.2 | 1.38 |
| 161 | 1753 | Liquid | 43.9 | 5.4 | 18.7 | 31.9 | 1.37 |
| 166 | 1753 | Liquid | 43.2 | 12.2 | 12.4 | 32.2 | 1.34 |
| 188 | 1753 | Liquid | 42.9 | 12.0 | 12.3 | 32.9 | 1.31 |
| 189 | 1753 | Liquid | 49.9 | 0.0 | 11.9 | 38.1 | 1.31 |
| 190 | 1753 | Liquid | 48.2 | 2.1 | 12.4 | 37.3 | 1.29 |
| 192 | 1753 | Liquid | 44.9 | 8.6 | 12.1 | 34.4 | 1.31 |
| 7 | 1773 | Liquid | 49.5 | 0.0 | 11.2 | 39.3 | 1.26 |
| 50 | 1773 | Liquid | 37.2 | 13.8 | 20.1 | 29.0 | 1.28 |
| 126 | 1773 | Liquid | 41.5 | 5.0 | 21.2 | 32.2 | 1.29 |
| 63 | 1773 | Liquid | 38.6 | 7.6 | 24.0 | 29.8 | 1.30 |
| 62 | 1773 | Liquid | 40.0 | 6.9 | 22.4 | 30.7 | 1.30 |
| 78 | 1773 | Liquid | 44.0 | 9.9 | 12.4 | 33.7 | 1.30 |
| 127 | 1773 | Liquid | 38.6 | 6.0 | 26.0 | 29.5 | 1.31 |
| 79 | 1773 | Liquid | 42.8 | 12.1 | 12.5 | 32.6 | 1.31 |
| 97 | 1773 | Liquid | 38.2 | 6.9 | 25.8 | 29.0 | 1.32 |
| 96 | 1773 | Liquid | 39.6 | 6.0 | 24.3 | 30.0 | 1.32 |
| 128 | 1773 | Liquid | 36.5 | 5.9 | 30.0 | 27.6 | 1.32 |
| 110 | 1773 | Liquid | 36.7 | 7.5 | 28.1 | 27.6 | 1.33 |
| 137 | 1773 | Liquid | 38.0 | 3.9 | 29.6 | 28.5 | 1.33 |
| 43 | 1773 | Liquid | 38.6 | 8.4 | 24.7 | 28.3 | 1.36 |
| Liquid with one oxide solid | |||||||
| Meliite primary phase field | |||||||
| 15 | 1673 | Liquid | 47.4 | 0.0 | 13.6 | 39.0 | 1.21 |
| Melilite | 41.1 | 0.0 | 36.5 | 22.3 | |||
| 8 | 1673 | Liquid | 45.5 | 5.6 | 11.0 | 37.9 | 1.20 |
| Melilite | 41.0 | 5.8 | 22.5 | 30.8 | |||
| 21 | 1703 | Liquid | 47.4 | 0.0 | 15.1 | 37.5 | 1.26 |
| Melilite | 41.5 | 0.0 | 36.4 | 22.2 | |||
| 12 | 1723 | Liquid | 45.9 | 0.0 | 16.7 | 37.4 | 1.23 |
| Melilite | 41.0 | 0.0 | 36.8 | 22.1 | |||
| 9 | 1723 | Liquid | 44.6 | 5.4 | 14.5 | 35.5 | 1.26 |
| Melilite | 41.0 | 2.8 | 29.8 | 26.3 | |||
| 123 | 1723 | Liquid | 39.4 | 10.9 | 18.3 | 31.3 | 1.26 |
| Melilite | 41.0 | 2.8 | 30.1 | 26.1 | |||
| 52 | 1723 | Liquid | 44.0 | 7.5 | 14.5 | 34.0 | 1.29 |
| Melilite | 41.3 | 3.0 | 28.8 | 26.9 | |||
| 133 | 1723 | Liquid | 48.0 | 0.0 | 14.9 | 37.1 | 1.29 |
| Melilite | 41.0 | 0.0 | 36.9 | 22.1 | |||
| 132 | 1723 | Liquid | 47.4 | 2.0 | 14.1 | 36.5 | 1.30 |
| Melilite | 40.9 | 1.4 | 33.4 | 24.2 | |||
| 122 | 1723 | Liquid | 41.9 | 9.7 | 16.2 | 32.2 | 1.30 |
| Melilite | 40.9 | 2.9 | 29.9 | 26.4 | |||
| 39 | 1723 | Liquid | 48.8 | 0.0 | 14.4 | 36.7 | 1.33 |
| Melilite | 40.9 | 0.0 | 37.2 | 21.9 | |||
| 41 | 1723 | Liquid | 42.8 | 9.3 | 15.8 | 32.1 | 1.33 |
| Melilite | 41.2 | 2.7 | 30.3 | 25.8 | |||
| 22 | 1733 | Liquid | 46.3 | 0.0 | 16.9 | 36.8 | 1.26 |
| Melilite | 41.3 | 0.0 | 36.5 | 22.2 | |||
| 119 | 1753 | Liquid | 38.0 | 9.6 | 23.5 | 29.0 | 1.31 |
| Melilite | 41.1 | 1.8 | 32.8 | 24.3 | |||
| 163 | 1753 | Liquid | 47.6 | 0.0 | 17.8 | 34.6 | 1.37 |
| Melilite | 42.0 | 0.0 | 36.4 | 21.6 | |||
| 181 | 1753 | Liquid | 45.8 | 0.0 | 18.2 | 36.0 | 1.28 |
| Melilite | 41.4 | 0.0 | 36.6 | 21.9 | |||
| 182 | 1753 | Liquid | 44.9 | 1.8 | 18.2 | 35.1 | 1.28 |
| Melilite | 40.9 | 0.9 | 34.6 | 23.6 | |||
| 183 | 1753 | Liquid | 44.1 | 3.5 | 17.6 | 34.9 | 1.26 |
| Melilite | 41.3 | 1.5 | 32.9 | 24.3 | |||
| 184 | 1753 | Liquid | 42.6 | 5.2 | 18.3 | 33.9 | 1.26 |
| Melilite | 41.1 | 1.8 | 32.3 | 24.8 | |||
| 185 | 1753 | Liquid | 40.5 | 7.1 | 19.7 | 32.7 | 1.24 |
| Melilite | 41.1 | 2.0 | 31.9 | 25.0 | |||
| 186 | 1773 | Liquid | 34.3 | 0.0 | 36.5 | 29.2 | 1.18 |
| Melilite | 41.1 | 0.0 | 37.2 | 21.7 | |||
| 164 | 1773 | Liquid | 37.1 | 0.0 | 31.6 | 31.3 | 1.19 |
| Melilite | 41.9 | 0.0 | 36.5 | 21.6 | |||
| 138 | 1773 | Liquid | 40.8 | 4.8 | 20.8 | 33.5 | 1.22 |
| Melilite | 41.1 | 1.7 | 32.8 | 24.4 | |||
| 6 | 1773 | Liquid | 43.4 | 0.0 | 21.6 | 35.0 | 1.24 |
| Melilite | 41.0 | 0.0 | 37.2 | 21.9 | |||
| 46 | 1773 | Liquid | 45.1 | 0.0 | 19.9 | 35.0 | 1.29 |
| Melilite | 40.9 | 0.0 | 37.3 | 21.8 | |||
| 125 | 1773 | Liquid | 44.5 | 2.1 | 19.4 | 34.0 | 1.31 |
| Melilite | 41.2 | 0.9 | 34.6 | 23.3 | |||
| 42 | 1773 | Liquid | 42.4 | 5.4 | 20.3 | 31.9 | 1.33 |
| Melilite | 40.8 | 1.6 | 33.3 | 24.3 | |||
| Merwinite primary phase field | |||||||
| 69 | 1723 | Liquid | 43.1 | 9.6 | 13.8 | 33.4 | 1.29 |
| Merwinite | 51.3 | 12.1 | 0.1 | 36.5 | |||
| 70 | 1723 | Liquid | 40.6 | 12.1 | 15.4 | 31.9 | 1.27 |
| Merwinite | 51.0 | 12.3 | 0.1 | 36.6 | |||
| 92 | 1773 | Liquid | 43.3 | 12.5 | 11.3 | 32.8 | 1.32 |
| Merwinite | 51.3 | 12.3 | 0.1 | 36.3 | |||
| Dicalcium silicate primary phase field | |||||||
| 115 | 1673 | Liquid | 43.8 | 8.2 | 13.2 | 34.9 | 1.26 |
| Dicalcium silicate | 60.7 | 3.1 | 0.3 | 36.1 | |||
| 112 | 1723 | Liquid | 48.3 | 0.0 | 12.8 | 38.9 | 1.24 |
| Dicalcium silicate | 63.7 | 0.0 | 0.4 | 35.9 | |||
| 24 | 1723 | Liquid | 45.3 | 10.6 | 7.4 | 36.8 | 1.23 |
| Dicalcium silicate | 60.3 | 4.1 | 0.2 | 35.4 | |||
| 86 | 1723 | Liquid | 46.7 | 4.6 | 11.8 | 36.8 | 1.27 |
| Dicalcium silicate | 62.9 | 1.8 | 0.3 | 35.0 | |||
| 85 | 1723 | Liquid | 48.3 | 2.3 | 11.6 | 37.9 | 1.27 |
| Dicalcium silicate | 63.8 | 1.0 | 0.4 | 34.8 | |||
| 129 | 1723 | Liquid | 44.3 | 8.0 | 13.3 | 34.4 | 1.29 |
| Dicalcium silicate | 61.4 | 3.0 | 0.2 | 35.3 | |||
| 76 | 1723 | Liquid | 44.7 | 7.6 | 12.9 | 34.8 | 1.29 |
| Dicalcium silicate | 61.4 | 2.8 | 0.2 | 35.5 | |||
| 99 | 1723 | Liquid | 45.6 | 5.9 | 13.3 | 35.2 | 1.30 |
| Dicalcium silicate | 62.1 | 2.3 | 0.3 | 35.3 | |||
| 131 | 1723 | Liquid | 48.4 | 0.0 | 13.6 | 37.9 | 1.28 |
| Dicalcium silicate | 64.6 | 0.0 | 0.4 | 35.0 | |||
| 117 | 1723 | Liquid | 44.3 | 8.1 | 14.0 | 33.7 | 1.31 |
| Dicalcium silicate | 61.9 | 3.0 | 0.2 | 34.9 | |||
| 38 | 1723 | Liquid | 46.3 | 5.2 | 13.6 | 34.9 | 1.33 |
| Dicalcium silicate | 62.8 | 2.0 | 0.3 | 34.9 | |||
| 191 | 1723 | Liquid | 48.1 | 3.2 | 12.7 | 36.0 | 1.34 |
| Dicalcium silicate | 64.4 | 1.0 | 0.3 | 34.3 | |||
| 98 | 1773 | Liquid | 44.0 | 11.6 | 8.6 | 35.7 | 1.23 |
| Dicalcium silicate | 60.3 | 4.2 | 0.1 | 35.2 | |||
| 95 | 1773 | Liquid | 44.8 | 8.6 | 10.5 | 36.1 | 1.24 |
| Dicalcium silicate | 61.3 | 3.2 | 0.2 | 35.2 | |||
| 94 | 1773 | Liquid | 47.2 | 4.5 | 11.1 | 37.2 | 1.27 |
| Dicalcium silicate | 62.9 | 1.6 | 0.3 | 35.1 | |||
| 93 | 1773 | Liquid | 48.5 | 2.3 | 11.0 | 38.2 | 1.27 |
| Dicalcium silicate | 63.9 | 0.8 | 0.3 | 35.0 | |||
| 89 | 1773 | Liquid | 44.5 | 10.5 | 10.0 | 35.0 | 1.27 |
| Dicalcium silicate | 60.7 | 3.9 | 0.2 | 35.3 | |||
| 90 | 1773 | Liquid | 44.3 | 11.1 | 10.0 | 34.6 | 1.28 |
| Dicalcium silicate | 60.5 | 3.9 | 0.2 | 35.4 | |||
| 106 | 1773 | Liquid | 44.4 | 10.2 | 11.3 | 34.2 | 1.30 |
| Dicalcium silicate | 61.0 | 3.5 | 0.2 | 35.2 | |||
| 105 | 1773 | Liquid | 47.0 | 5.2 | 11.6 | 36.2 | 1.30 |
| Dicalcium silicate | 62.8 | 1.9 | 0.2 | 35.0 | |||
| 103 | 1773 | Liquid | 50.2 | 0.0 | 11.2 | 38.5 | 1.30 |
| Dicalcium silicate | 64.8 | 0.0 | 0.4 | 34.9 | |||
| Spinel primary phase field | |||||||
| 66 | 1723 | Liquid | 39.3 | 13.0 | 17.2 | 30.5 | 1.29 |
| Spinel | 0.5 | 28.0 | 71.0 | 0.5 | |||
| 54 | 1723 | Liquid | 39.5 | 12.4 | 17.5 | 30.6 | 1.29 |
| Spinel | 0.4 | 28.1 | 71.0 | 0.5 | |||
| 165 | 1753 | Liquid | 38.6 | 10.6 | 22.2 | 28.7 | 1.35 |
| Spinel | 0.2 | 28.1 | 71.7 | 0.0 | |||
| 187 | 1753 | Liquid | 37.9 | 13.9 | 18.3 | 29.9 | 1.27 |
| Spinel | 0.1 | 28.1 | 71.7 | 0.1 | |||
| 18 | 1773 | Liquid | 36.5 | 9.6 | 24.8 | 29.1 | 1.26 |
| Spinel | 0.6 | 28.2 | 70.9 | 0.4 | |||
| 44 | 1773 | Liquid | 37.9 | 9.5 | 24.6 | 27.9 | 1.36 |
| Spinel | 1.0 | 27.9 | 70.4 | 0.7 | |||
| 45 | 1773 | Liquid | 38.1 | 11.5 | 22.6 | 27.9 | 1.36 |
| Spinel | 0.5 | 27.6 | 71.1 | 0.8 | |||
| 136 | 1773 | Liquid | 36.6 | 7.0 | 28.6 | 27.8 | 1.32 |
| Spinel | 0.1 | 28.0 | 71.9 | 0.0 | |||
| Periclase (MgO) primary phase field | |||||||
| 58 | 1723 | Liquid | 39.3 | 14.5 | 15.6 | 30.7 | 1.28 |
| Periclase | 0.3 | 98.9 | 0.9 | 0.0 | |||
| 167 | 1753 | Liquid | 40.4 | 14.9 | 14.3 | 30.4 | 1.33 |
| Periclase | 0.3 | 98.9 | 0.8 | 0.0 | |||
| 57 | 1773 | Liquid | 38.0 | 15.9 | 16.4 | 29.7 | 1.28 |
| Periclase | 0.3 | 98.7 | 1.0 | 0.0 | |||
| 71 | 1773 | Liquid | 39.9 | 15.0 | 14.3 | 30.9 | 1.29 |
| Periclase | 0.3 | 98.8 | 0.8 | 0.1 | |||
| 80 | 1773 | Liquid | 41.4 | 14.6 | 12.3 | 31.7 | 1.31 |
| Periclase | 0.3 | 99.0 | 0.7 | 0.0 | |||
| Liquid with more oxide solids | |||||||
| 82 | 1723 | Liquid | 44.6 | 8.5 | 11.8 | 35.1 | 1.27 |
| Dicalcium silicate | 61.3 | 3.2 | 0.2 | 35.2 | |||
| Merwinite | 51.6 | 12.0 | 0.1 | 36.3 | |||
| 87 | 1723 | Liquid | 44.6 | 8.4 | 11.7 | 35.3 | 1.26 |
| Dicalcium silicate | 61.3 | 3.3 | 0.2 | 35.2 | |||
| Merwinite | 51.5 | 12.2 | 0.1 | 36.3 | |||
| 88 | 1723 | Liquid | 44.4 | 8.6 | 12.1 | 35.0 | 1.27 |
| Dicalcium silicate | 61.5 | 3.3 | 0.2 | 35.0 | |||
| Merwinite | 51.4 | 12.2 | 0.1 | 36.4 | |||
| 91 | 1773 | Liquid | 43.7 | 11.9 | 10.7 | 33.6 | 1.30 |
| Dicalcium silicate | 60.4 | 4.3 | 0.2 | 35.3 | |||
| Merwinite | 51.3 | 12.1 | 0.1 | 36.4 | |||
| 102 | 1693 | Liquid | 45.6 | 5.8 | 11.2 | 37.4 | 1.22 |
| Dicalcium silicate | 62.0 | 2.3 | 0.3 | 35.3 | |||
| Melilite | 45.2 | 3.8 | 21.4 | 29.6 | |||
| 59 | 1673 | Liquid | 45.4 | 6.3 | 9.7 | 38.6 | 1.18 |
| Dicalcium silicate | 61.5 | 2.7 | 0.3 | 35.5 | |||
| Melilite | 41.2 | 6.5 | 20.1 | 32.3 | |||
| 55 | 1673 | Liquid | 47.4 | 2.7 | 11.0 | 39 | 1.22 |
| Dicalcium silicate | 63.3 | 1.1 | 0.4 | 35.1 | |||
| Melilite | 41.4 | 2.9 | 29.2 | 26.5 | |||
| 5 | 1673 | Liquid | 48.3 | 0.0 | 12.6 | 39 | 1.24 |
| Dicalcium silicate | 65.2 | 0.0 | 0.0 | 34.8 | |||
| Melilite | 40.9 | 0.0 | 36.7 | 22.4 | |||
| 107 | 1723 | Liquid | 46.1 | 5.2 | 13.2 | 35.4 | 1.30 |
| Dicalcium silicate | 62.6 | 2.1 | 0.3 | 35.1 | |||
| Melilite | 41.0 | 2.8 | 30.1 | 26.1 | |||
| 72 | 1723 | Liquid | 38.6 | 14.8 | 16.1 | 30.6 | 1.26 |
| Periclase | 0.5 | 98.9 | 0.5 | 0.1 | |||
| Merwinite | 50.9 | 12.4 | 0.1 | 36.6 | |||
| 81 | 1773 | Liquid | 42.0 | 14.4 | 11.2 | 32.5 | 1.29 |
| Periclase | 0.3 | 99.0 | 0.6 | 0.0 | |||
| Merwinite | 51.1 | 12.3 | 0.1 | 36.5 | |||
| 77 | 1723 | Liquid | 39.4 | 14.5 | 15.7 | 30.4 | 1.30 |
| Periclase | 0.3 | 98.9 | 0.8 | 0.0 | |||
| Merwinite | 50.9 | 12.4 | 0.1 | 36.6 | |||
| 116 | 1723 | Liquid | 39.2 | 11.5 | 18.3 | 31.0 | 1.26 |
| Spinel | 0.7 | 28.0 | 70.9 | 0.4 | |||
| Melilite | 40.7 | 2.5 | 31.1 | 25.7 | |||
| 134 | 1693 | Liquid | 45.1 | 7.1 | 11.0 | 36.9 | 1.22 |
| Merwinite | 51.6 | 12.0 | 0.1 | 36.3 | |||
| Melilite | 41.3 | 5.6 | 22.6 | 30.5 | |||
| 111 | 1703 | Liquid | 40.0 | 11.3 | 14.7 | 34 | 1.18 |
| Merwinite | 50.3 | 12.4 | 0.1 | 37.1 | |||
| Melilite | 40.5 | 4.2 | 26.5 | 29.0 | |||
| Dicalcium silicate | 64.2 | 0.0 | 0.4 | 35.4 | |||
| 101 | 1723 | Liquid | 37.7 | 11.8 | 19.3 | 31.1 | 1.21 |
| Spinel | 0.1 | 28.2 | 71.7 | 0.0 | |||
| Merwinite | 40.8 | 2.9 | 30.1 | 26.3 | |||
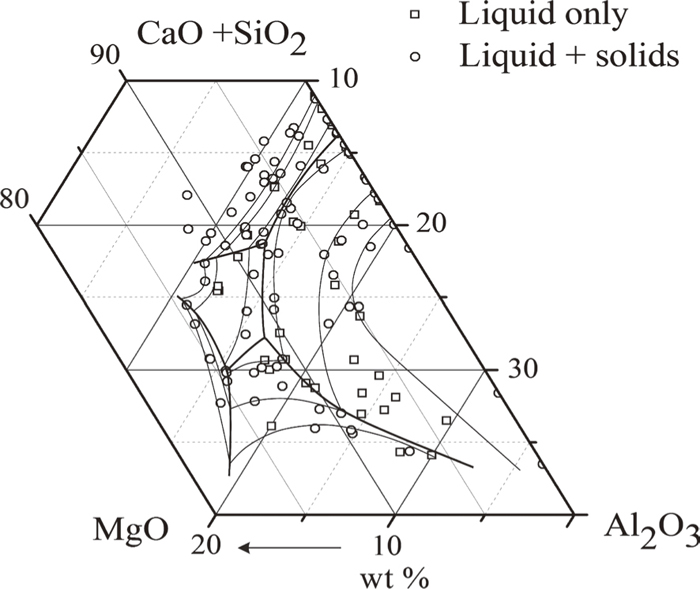
Experimental composition range in the CaO–SiO2–MgO–Al2O3 system.

Typical microstructures of slags quenched from (a) melilite, (b) Ca2SiO4 and (c) spinel primary phase fields and (d) merwinite and Ca2SiO4 phase boundary.
The data given in Table 1 have been used to construct liquidus isotherms of the pseudo-ternary section (CaO+SiO2)–Al2O3–MgO with a fixed CaO/SiO2 weight ratio of 1.30, as shown in Fig. 4. The results reported by Osborn et al.,5) Cavalier and Sandrea-Deudon,7) Muan and Osborn18) are also given in Fig. 4 for comparison. The present experimental results show general agreement with previous works. The liquidus temperatures in Ca2SiO4 primary phase field reported by Osborn et al.5) are much higher than the present results. In addition, predictions of FactSage 6.219) are also shown in the figure for comparison. The databases selected in FactSage 6.2 are “Fact53” and “FToxide”, and the solutions species selected in calculation are “FToxide-SLAGA”, “FToxide-SPINA”, “FToxide-MeO_A”, “FToxide-bC2S”, “FToxide-aC2S”, “FToxide-Mel_”, and “FToxide-Merwinite”. It can be seen that FactSage predictions show the similar trends as the experimental results, but the locations of the isotherms are significantly different. Experimentally determined liquidus temperatures in the spinel and Ca2SiO4 primary phase fields are approximately 50 K lower than those predicted by FactSage 6.2. However, in merwinite primary phase field, the experimentally determined liquidus temperatures are approximately 50 K higher than the predictions. A significant difference is that the experimentally determined merwinite phase area is larger than the FactSage predictions. This significant difference may come from the lack of the thermodynamics data in merwinite primary phase field. In melilite primary phase field, the experimentally determined liquidus temperatures are usually 20 K different from predicted values.

Pseudo-ternary section (CaO+SiO2)–Al2O3–MgO with CaO/SiO2 ratio of 1.30.
Melilite is the solid solution between akermanite (2CaO·MgO·2SiO2) and gehlenite (2CaO·Al2O3·SiO2). The distributions of MgO and Al2O3 between melilite and liquid are shown in Figs. 5 and 6 respectively. As seen in Fig. 5, the MgO concentrations in melilite solid solution first increase with increasing MgO concentration in liquid, and then slightly decrease after reaching the maximum. It is also found that the MgO concentrations in melilite decrease with increasing temperature and the predicted MgO concentrations in melilite by FactSage 6.2 are higher than the experimental results. As seen in Fig. 6, the distribution of Al2O3 between melilite and liquid is complicated. For a given Al2O3 concentration in the liquid, there are two corresponding melilite compositions. Generally the Al2O3 concentration in melilite is higher than that in the liquid phase. It is interesting to see that FactSage can predict the general trend of the distributions but the values are significantly different which can explain the difference in liquidus temperatures.

Distribution of MgO between melilite and liquid for temperature between 1723 and 1773 K.

Distribution of Al2O3 between melilite and liquid for temperature between 1723 and 1773 K.
In most case, the ironmaking BF slags have the CaO/SiO2 ratio in the range of 1.10 to 1.30 in practical operations. The isotherms in the CaO–SiO2–Al2O3–MgO system with CaO/SiO2 ratio of 1.10 reported by Zhang et al.14) are compared with the present work with CaO/SiO2 ratio of 1.30 as shown in Fig. 7. The melilite primary phase field dominates in the composition range studied at CaO/SiO2 ratio of 1.10. However, when the CaO/SiO2 ratio is increased to 1.30, dicalcium silicate becomes more stable and its primary phase field shifts towards high Al2O3 direction. The liquidus temperatures are also significantly affected with increasing CaO/SiO2 ratio and the details will be discussed in the following sections.

Comparison of the sections (CaO+SiO2)–Al2O3–MgO with CaO/SiO2 ratio of 1.30 and 1.10.14)
Recently BF operation is facing the challenges to lower the cost by the increase of pulverized coal injection, utilization of low-grade ores and reduction of slag volume. These changes could result in higher Al2O3 or lower MgO in the slag.20,21,22) Figures 8(a) and 8(b) show the relationship between the liquidus temperatures and MgO concentration at fixed Al2O3 of 15 and 20 wt%. 15 wt% Al2O3 in slag represents an average in the current operation and 20 wt% Al2O3 in slag is a result of low grade iron ore utilization. It can be seen from Fig. 8(a) that with 15 wt% Al2O3 in slag, melilite and MgO are the primary phases in the composition range up to 15 wt% MgO and CaO/SiO2 ratio of 1.1. The liquidus temperatures in MgO primary phase field increase dramatically with increasing MgO concentration. The MgO concentration (15 wt%) where MgO starts to form is the up limit. On the other hand, it can be seen that decrease of MgO from 10 wt% to 5 wt% can decrease the liquidus temperature by 40 K. At CaO/SiO2 ratio of 1.3 merwinite primary phase field appears between the melilite and MgO primary phase fields. The liquidus temperatures in the melilite primary phase field has a maximum at 7 wt% MgO. When low grade iron ore is used the Al2O3 concentration in the slag can be as high as 20 wt%. It can be seen from Fig. 8(b) that spinel phase will appear at high MgO concentration. The liquidus temperatures in the spinel primary phase field increase significantly with increasing MgO concentration. At high Al2O3 (20 wt%) and high CaO/SiO2 ratio (1.3) decrease of MgO from 10 to 5 wt% can cause significant increase of the liquidus temperature. The accurate information given here will provide useful guide to BF operators to work at temperatures high enough to avoid the precipitation of solid phases.

The effect of MgO on the liquidus temperature in which Al2O3 = 15 and 20 wt%.
Figures 9(a) and 9(b) show the relationship between the liquidus temperatures and Al2O3 concentration at fixed MgO of 5 and 10 wt% respectively. 10 wt% MgO in slag represents an average in the current operation and 5 wt% MgO in slag is a result of low slag rate operation. It can be seen that Al2O3 has strong influence in liquidus temperatures in all primary phase fields present in the composition range investigated. In the melilite and spinel primary phase fields the liquidus temperatures increase significantly with increasing Al2O3 concentration. In contrast, the liquidus temperatures in the dicalcium silicate and merwinite primary phase fields decrease significantly with increasing Al2O3 concentration.

The effect of Al2O3 on the liquidus temperature in which MgO = 5 and 10 wt%.
The phase equilibria and liquidus temperatures in the CaO–SiO2–Al2O3–MgO system with CaO/SiO2 ratio of 1.30 have been experimentally determined from 1723 to 1773 K. Effects of CaO/SiO2 ratio, Al2O3 and MgO concentrations on liquidus temperature have been presented. Extensive solid solutions of melilite have been accurately determined that will provide invaluable data for optimisation of thermodynamic database.
The authors would like to thank Ms. Jie Yu for the lab assistance in the high temperature experiments and financial support from Baosteel through The Baosteel-Australia Joint Research and Development Centre. The authors also would like to thank Mr Ron Rasch and Ms Ying Yu in Centre for Microscopy and Microanalysis (CMM) for technical support of EPMA and SEM.