2015 Volume 55 Issue 2 Pages 413-418
2015 Volume 55 Issue 2 Pages 413-418
Dissolved carbon in molten iron can be decarburized by CO2 gas instead O2 gas commonly used in a basic oxygen furnace, in which CO2 gas is reduced to CO gas. Produced CO gas can be returned to ironmaking process and as a whole carbon works as energy transfer medium. However, the precise control of the process is required to utilize the decarburization reaction by CO2–O2 gas mixture because of its significant endothermic reaction. The present study has focused on the decarburization reaction of molten Fe–C alloy by CO2–O2 or H2O–CO2–O2 gas mixtures and the effects of gas composition and temperature on temperature change of molten alloy and exhaust gas compositions were studied by means of thermodynamic calculation. Repeated equilibrium calculations between existing molten alloy and newly introduced reacting gas enabled the estimation of molten alloy temperature and exhaust gas composition changes. Results were discussed in terms of molten alloy temperature change and conversion ratio of CO2 to CO or H2O to H2.
More than 100 Mt of crude steel is annually produced in Japan1) and the emission of CO2 gas from steel industries accounts for around 12.5% of total domestic CO2 emission (FY2012).2) Therefore, the reduction in CO2 emission from integrated steel production process is responsible for the dramatic curtailment of an environmental load and the establishment of a sustainable steel production process. Various technologies for the recovery and storage of CO2 gas have been widely studied and pilot-scale plant tests have been conducted, one of which is COURSE50 project conducted by Japanese steelmaking companies with the support of New Energy and Industrial Technology Development Organization and the Japan Iron and Steel Federation.3) If recovered CO2 gas could be utilized effectively by circulating in ironmaking and steelmaking processes, CO2 gas could become an energy transfer medium. However, recovered CO2 gas has nearly no value as is and thus some sort of energy must be adequately given. One of such methods is the conversion of CO2 gas to valuable CO gas by the reduction reaction. The value of CO gas is relatively high as a fuel in steel plants or a reducing agent for newly developed various ironmaking processes. CO gas is also required in various petroleum refinery processes and chemical plants.
Many processes to reduce CO2 gas to CO gas have been proposed, such as electrolysis, hydrogen reduction, and thermo chemical reduction. In the present study, the possibility of the reduction of CO2 gas to CO gas in the steelmaking process has been considered, one of which is the utilization of CO2 gas as an oxidizing gas for the decarburization process. Usually O2 gas is used to decarburize hot metal to produce molten steel, which reaction is expressed by Eq. (1).
| (1) 4) |
On the contrary, the decarburization reaction by CO2 gas is expressed as follows.
| (2) 5) |
In the present study, thermodynamic estimations of decarburization reaction by using CO2 gas were conducted with various operation conditions and the effects of such conditions on the decarburization reaction behavior, degree of the reduction of CO2 gas to CO gas and temperature variation were studied.
In the present study, thermodynamic calculation software FactSage 6.3.1 was used for estimation of equilibrium state between molten Fe–C alloy and CO2-containing gas.
Table 1 shows the detail of calculation conditions. Firstly, 1 kg of molten Fe–3.5 mass%C alloy was prepared at 1573 K. Subsequently the prepared molten Fe–C alloy was equilibrated with O2, CO2–O2 or CO2–H2O–O2 gas in various compositions and temperatures. In the equilibrium calculation between molten Fe–C alloy and gas, 87 kinds of pure compounds (pure gaseous species in ideal behavior: 43, pure liquid species: 16 and pure solid species: 28) and a solution considering 3 species were considered in the equilibrium calculation as final candidates. For the estimation of change in temperature and composition of molten Fe–C alloy and exhaust gas with proceeding of decarburization reaction, 1.0 L of reacting gas at predetermined gas temperature was equilibrated with molten Fe–C alloy. The amount, temperature and composition of molten Fe–C alloy after equilibrium calculation were input as the initial alloy conditions in the next calculation, and 1.0 L of new reacting gas was equilibrated again. The above equilibrium calculation between molten Fe–C alloy and gas was repeated by decreasing carbon content of Fe–C alloy less than 0.1 mass%. Change in alloy and exhaust gas compositions and system temperature with process of reaction between molten Fe–C alloy and reacting gas were estimated. The algorithm of equilibrium calculation is schematically shown in Fig. 1.
| Molten Fe–C alloy preparation | |
| Reaction | Fe (s) + C (s) → Fe–C (l) |
| Amount | Total: 1 kg |
| Number of compounds | 0 |
| Number of solutions | 1 (Species: 2) |
| FSstel-LIQU (Metal-liquid) | |
| Temperature | Fixed (1573 K) |
| Composition | C: 3.5 mass% |
| Alloy – gas equilibrium | |
| Reaction | Fe–C (l) + H2O–CO2–O2 (g) → Fe–C (l) + H2O–H2–CO2–CO–O2 (g) |
| Amount | Molten Fe–C alloy + 1.0 L of gas (1 calculation) |
| Number of compounds | Total: 87 (Gas(ideal): 43, Liquid: 16, Solid: 28) |
| Number of solutions | 1 (Species: 3) |
| FSstel-LIQU (Metal-liquid) | |
| Gas temperature | 300–1500 K |
| Gas composition | P(CO2): 0.0–0.8 atm, P(H2O): 0.0–0.2 atm |
| Temperature | Change to satisfy 80% thermal efficiency |

Algorithm of equilibrium calculation.
As explained previously, one of objectives in the present study is the estimation of temperature change of molten steel during decarburization reaction by CO2-containing gas. However, the present calculation method using thermodynamic equilibrium has a limitation for the consideration of dynamic change of BOF steelmaking process such as scrap melting or slag formation. Therefore, the present method simply calculated the equilibrium between molten Fe–C alloy and blowing gas and thus thermal energy is excess compared to an actual operation. To estimate reasonable temperature change during calculation, heat utilization efficiency was preliminary determined. Figure 2 shows the effect of thermal efficiency on temperature change of the system during equilibrium calculation between molten Fe–C alloy and pure O2 gas introduced at 300 K, in which 100% thermal efficiency means that the equilibrium calculation is processed in the adiabatic condition. However, the adiabatic condition increased the system temperature above 2000 K when molten Fe–C alloy was decarburized from 3.5 mass%C to 0.1 mass%C, which is unrealistic. As shown in the figure, molten steel temperature reaches around 1920 K when thermal efficiency is 80%, which is close to molten steel temperature at the blow-end. Therefore, the thermal efficiency in following all calculations was set as 80%, which means that 80% of thermal energy generated by decarburization reaction is used to increase the system temperature and 20% is simply lost.

Effect of thermal efficiency on temperature change of the system during decarburization reaction of molten Fe–C alloy.
Equilibrium calculation between molten Fe–C alloy and CO2–O2 gas introduced at 300 K was conducted by changing CO2 gas composition. Figure 3 shows the effect of partial pressure of CO2 gas in CO2–O2 gas on temperature change of the system during decarburization of molten Fe–C alloy. Temperature change became smaller with increasing CO2 content of introduced gas. Temperature and carbon content of molten Fe–C alloy reached the liquidus of the Fe–C system in the case CO2 partial pressure more than 0.3 atm, in which solid Fe was precipitated during decarburization reaction. Molten alloy temperature even decreased with decreasing carbon content by CO2–O2 gas containing more than 60% of CO2. Therefore, CO2 content in CO2–O2 gas must be lower than 20% to avoid any solid steel precipitation.

Effect of partial pressure of CO2 on temperature change of the system during decarburization reaction of molten Fe–C alloy.
Figure 4 shows the change in partial pressures of CO and CO2 in exhaust gas with carbon content of molten Fe–C alloy. Partial pressure of CO gas is close to 1 atm and the effect of CO2 partial pressure in introduced gas was not clearly seen. On the contrary, partial pressure of CO2 gas gradually increased with the decrease of carbon content. The increasing trend became significant at carbon content below 1 mass%. Decrease in carbon content of molten Fe–C alloy decreases activity of carbon and thus the reduction degree of CO2 gas became lower at lower carbon content region as expected from the equilibrium constant of reaction (2).

Effect of partial pressure of CO2 on the relationship between partial pressures of CO and CO2 and carbon content in molten Fe–C alloy.
Instantaneous and integrated conversion ratios of CO2 gas to CO gas were calculated by Eqs. (3) and (4), respectively.
| (3) |
| (4) |

Change in conversion ratio of CO2 to CO with (a) carbon content and (b) introduced CO2 amount during decarburization reaction of molten Fe–C alloy.
Equilibrium calculation between molten Fe–C alloy and 0.2 atm CO2–O2 gas was conducted by changing introduced gas temperature. Figure 6 shows the relationship between carbon content of molten Fe–C alloy and the system temperature. Molten Fe–C alloy temperature at the blow-end increased by 60 K from 1820 K to 1880 K by increasing gas temperature from 300 K to 1500 K.
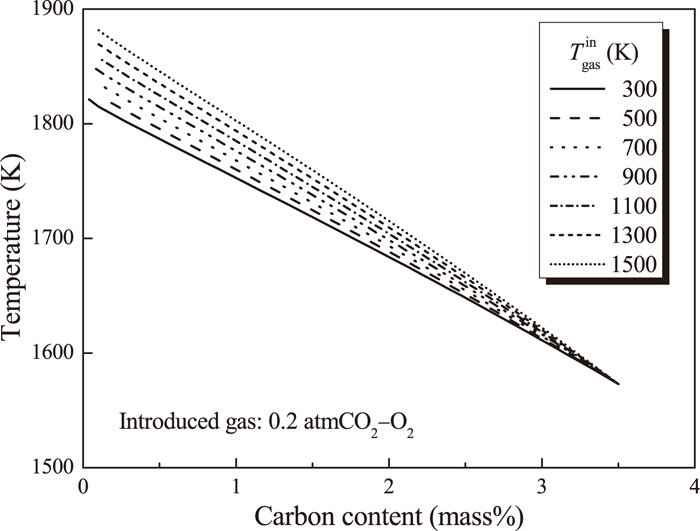
Effect of introduced gas temperature on temperature change of the system during decarburization reaction of molten Fe–C alloy.
Figure 7 shows the effect of introduced gas temperature on the change in partial pressures of CO and CO2 in exhaust gas. Effect of introduced gas temperature was insignificant but the temperature increase slightly decreased the equilibrated CO2 partial pressure.

Effect of introduced gas temperature on the relationship between partial pressures of CO and CO2 and carbon content in molten Fe–C alloy.
Figure 8 shows change in conversion ratio of CO2 gas to CO gas with (a) carbon of molten Fe–C alloy or (b) introduced CO2 gas amount in mole. Effect of introduced gas temperature on CO2 gas conversion ratio was insignificant. From above results, the effect of preheating of CO2–O2 gas before blowing on temperature increase of molten steel at the blow-end is limited and the positive effects on CO2 gas conversion ratio was not obviously observed.

Change in conversion ratio of CO2 to CO with (a) carbon content and (b) introduced CO2 amount during decarburization reaction of molten Fe–C alloy.
Similar to CO2 gas, H2O gas (water vapor) can also decarburize molten Fe–C alloy which reaction is expressed as Eq. (5).
| (5) 6) |
Equilibrium calculation between molten Fe–C alloy and x atm H2O – (0.2−x) atm CO2 – O2 gas (0 ≤ x ≤ 0.2) at 1500 K was conducted by changing partial pressure of H2O gas. Figure 9 shows the effect of H2O gas addition on temperature change of the system during decarburization reaction. Temperature change with decreasing carbon content in the case of H2O–CO2–O2 gas is similar to that of CO2–O2 gas and temperature slightly increased with increasing partial pressure of H2O gas. This is because of slightly smaller enthalpy change of Eq. (5) compared to that of Eq. (2). Temperature increase at the blow-end is however only 10 K when 0.2 atm CO2 gas is fully substituted by 0.2 atm H2O gas.

Effect of H2O addition into introduced gas on temperature change of the system during decarburization reaction of molten Fe–C alloy.
Change in partial pressures of CO, CO2, H2 and H2O with carbon content in molten Fe–C alloy is shown in Fig. 10. Partial pressure of CO2 is similar to that in the case of CO2–O2 gas equilibrium as shown in Fig. 7 and the effect of H2O gas addition is negligibly small. On the contrary, partial pressures of H2O and H2 obviously increased with increasing H2O partial pressure of introduced gas. Similar to CO2 partial pressure change, H2O partial pressure gradually increased with decreasing carbon content and the partial pressure ratio of H2 to H2O drastically decreased when carbon content decreased below 1 mass%.

Effect of H2O addition into introduced gas on the relationship between (a) partial pressures of CO and CO2 and carbon content in molten Fe–C alloy, and (b) partial pressures of H2 and H2O and carbon content in molten Fe–C alloy.
Instantaneous and integrated conversion ratios of H2O gas to H2 gas were calculated by Eqs. (6) and (7), respectively.
| (6) |
| (7) |

Change in conversion ratio of (a) CO2 to CO and (b) H2O to H2 with carbon content during decarburization reaction of molten Fe–C alloy by H2O–CO2–O2 gas.

Change in conversion ratio of (a) CO2 to CO with introduced CO2 amount and (b) H2O to H2 with introduced H2O amount during decarburization reaction of molten Fe–C alloy by H2O–CO2–O2 gas.
On the contrary, conversion ratio of H2O to H2 was not considerably affected with changing H2O partial pressure of introduced gas and more than 93% of instantaneous conversion ratio was maintained until the blow-end as shown in Figs. 11(b) and 12(b). Integrated conversion ratio was significantly large, more than 99%. Simultaneous addition of CO2 and H2O to oxidizing gas would be feasible to diminish the decrease of temperature of molten steel and also to produce CO and H2 gas simultaneously, in which especially almost complete reduction of H2O gas to H2 is expected.
In the present study, the conversion process of CO2 gas to CO gas utilizing thermal and chemical energy of molten iron was considered and the effect of the substitution of O2 gas to CO2–O2 gas or H2O–CO2–O2 gas on decarburization behavior of molten Fe–C alloy and conversion efficiency was examined by thermodynamic calculation.
Due to largely endothermic reaction of decarburization by CO2 gas, considerable difference in temperature change was observed after the change of O2 gas to CO2–O2 gas. The maximal substitution ratio of O2 gas to CO2 gas was 20% to avoid the precipitation of solid Fe during decarburization. Integrated conversion ratio of CO2 to CO was more than 95%, while instantaneous conversion ratio of CO2 to CO decreased drastically when carbon content decreased below 1 mass%.
Preheating of introduced gas from 300 K to 1500 K was not significantly effective to improve temperature decrease of molten alloy, which increased molten alloy temperature at the blow-end by about 60 K.
Addition of H2O gas as a substitute of CO2 slightly increased molten alloy temperature at the blow-end, approximately 10 K, when 0.2 atm H2O–O2 gas was introduced at 1500 K. This slight temperature increase is due to smaller enthalpy change of decarburization reaction by H2O gas than that by CO2 gas. Conversion ratio of H2O to H2 was more than 99% and thus the simultaneous addition of CO2 and H2O to oxidizing gas would be feasible to diminish temperature drop of molten steel and to produce CO and H2 gas simultaneously.
This research was partially supported by the Arai Science and Technology Foundation. Authors greatly appreciate the financial support.