2015 Volume 55 Issue 3 Pages 559-563
2015 Volume 55 Issue 3 Pages 559-563
The steel industry is important for the development of several economic activities in the world. Unfortunately, the electric arc furnace (EAF) used in production generates byproducts such as dust, which must be treated and confined to prevent pollution. In this study, the alkaline properties of solutions prepared with EAF dust were evaluated for use in CO2 capture. A process in which dust solution properties are regarded as an advantage is proposed. The results indicate that the developed process effectively minimizes operational problems. Specifically, the process neutralizes several hazardous pollutants contained in EAF dust, reduces CO2 emissions, and directly removes dioxin-forming dust from the exit gas stream.
The electric arc furnaces (EAFs) used in the steelmaking industry can generate dust volumes of up to 10–20 kg per ton of steel produced,1) and the global EAF dust production is estimated at around 3.7 million tons per year.2) EAF dust is primarily used as a reprocessing material for zinc recovery or as a raw material for the construction industry. A significant problem is that dust not-compliant to specifications should be confined, with high costs.
An EAF is a flexible piece of equipment for steelmaking because it can melt scrap metal and/or direct reduction iron into steel products with several mass ratios. Given the increased focus on improving EAF technology in the past 30 years, the use of such equipment requires an investment cost that is less than that required for a blast furnace.3,4) An EAF produces three main waste products: (1) gas, (2) slag, and (3) dust. Gases are produced by oxygen-induced chemical reactions in the EAF (e.g., carbon and natural gas combustion); at ~1873 K, the gases exit the EAF as CO2, CO, N2, and O2 as main components.2) Slag is produced during the melting process that generates a molten mixture of oxides (mainly Ca, Mg, Si and Fe oxides); because of its density, the mixture remains in the upper layer of the liquid steel bath.5) Slag has a dual function in the melting process: (a) It controls the chemical composition of steel via the additives that affect the chemical equilibrium between steel and slag, and (b) as slag foam, it covers the electric arc, thereby decreasing the energy lost through radiation.5)
The dust produced during EAF operation is transported with the gases from the EAF via an extraction system and then recovered in a baghouse located downstream. The physical properties and chemical composition of the dust depend on the raw materials used in the process. For instance, when galvanized scrap is used as feedstock for EAF steel production, most of the zinc from the steel scrap is carried into dust because of the very low solubility of zinc in molten steel and slag and because zinc vapor pressure is higher than iron vapor pressure at steelmaking temperatures (~1873 K). The chemical composition of the dust produced comprises simple and complex oxides of metals such as Fe, Ca, Na, Mg, Mn, Si, etc.,6,7) whose origins have been previously reported.8) EAF dust is a major issue in the production of industrial byproducts because it is difficult to recycle or reuse. As indicated in previous research,9) less than 25% of EAF dust is used for zinc recovery or fertilizer production. An economically viable zinc recovery process necessitates dust with high zinc content (>20 wt%).9,10,11,12,13,14)
Most EAF dust is disposed of in landfills, but this approach is an unsuitable long-term solution because it presents environmental and management issues. EAF dust also contains hazardous elements (zinc, lead, cadmium, chromium, arsenic, etc.) that are unacceptable in landfills without previous treatment. To address these problems, researchers have proposed treatment measures, such as chemical stabilization or ferrous and nonferrous metal recovery (e.g., pyrometallurgical or hydrometallurgical processes).9,10,15,16,17,18,19) Furthermore, EAF dust is involved in the formation of hazardous Cl-compounds, such as polychlorinated dibenzodioxins, polychlorinated dibenzofurans, and hexachlorobenzene.6) The formation these compounds can be decreased or prevented by dust removal from gases.
The discussion above shows that further investigation is needed, particularly on EAF dust processing because such treatment reduces or eliminates the problems associated with dust recycling or reuse, as well as its adverse effects on the steelmaking industry. Although EAF dust possesses harmful components, it also comprises substances such as calcium, sodium, potassium, and magnesium. These components have received less attention, yet they exhibit interesting properties that may be advantageous to steel production. For example, the interaction between EAF dust and water or wet air produces high-pH (up to 14) alkaline solutions that produce a high environmental impact. Motivated by this advantage, this study aims to measure the alkali content of several aqueous solutions synthesized with EAF dust and demonstrate the CO2 capture properties of the solutions. We also propose a simple process for neutralizing the harmful pollutants in EAF dust.
The potential of dust as a component in the production of alkaline solutions was determined by preparing solutions with a 5–35 wt% composition of dust in water, in accordance with the combinations in Table 1. A dust sample from a baghouse was used. The elemental analysis is presented in Table 2. The alkaline solutions were synthesized using the corresponding mass of dust and deionized water under magnetic stirring for 1 h, and then the pH was determined by a Hanna Instruments pH211 microprocessor.
| Sample | Wt. of dust | Wt. of water | pH measured (fresh solution) | OH– concentration of fresh solution (from pH measurement) (mmol/l) | OH– concentration of fresh solution (from pH estimation by ASPEN) (mmol/l) | Concentration of bases (from titration of fresh solution) (meq/l) |
|---|---|---|---|---|---|---|
| 1 | 5 | 95 | 12.87 | 74.1 | 144.4 | 90.0 |
| 2 | 10 | 90 | 12.95 | 89.1 | 297.6 | 116.7 |
| 3 | 15 | 85 | 13.22 | 165.9 | 470.4 | 250.0 |
| 4 | 20 | 80 | 13.15 | 141.2 | 665.0 | 400.0 |
| 5 | 25 | 75 | 13.24 | 173.7 | 886.0 | 526.7 |
| 6 | 30 | 70 | 13.21 | 162.2 | 1140.4 | 533.3 |
| 7 | 35 | 65 | 13.26 | 181.9 | 1439.6 | 600.0 |
| Element | mg/kg |
|---|---|
| Al | 2686 |
| Ba | 34.1 |
| Ca | 31200 |
| Cd | 59.3 |
| Cu | 374 |
| Cr | 435 |
| Fe | 394700 |
| K | 41030 |
| Li | 20.3 |
| Mg | 24890 |
| Na | 36790 |
| Sr | 30 |
| Ti | 526 |
| V | 274 |
| Zn | 118700 |
| Pb | 716 |
The OH– concentration was estimated by two methods: 1) from the direct pH measurements and the following equations:
| (1) |
| (2) |
| (3) |
Before the pH measurement, the alkaline solutions were neutralized by titration with HCl aqueous solution (1 M). In a conventional procedure, 100 ml of the alkaline solution was poured in an Erlenmeyer flask and then the HCl solution was added by dripping until a pH of 7 was reached, finally the solutions were aged for 24 h. after the 24 h aging, the pH was re-measured and if the pH was higher than 7 the dust solutions were re-titrated. This procedure was repeated 2–4 times until dust solutions with pH 7 or lower were derived.
To measure CO2 absorption capability, the dust solutions were placed in a 1000 ml bubble column to which an air–CO2 mixture (30% CO2 v/v) was fed at 298 K. The CO2 concentration of the gas mixture was measured by a gas chromatograph (GC, Perkin Elmer Clarus 400) equipped with a TCD detector, prior to the CO2 absorption experiments, a series of samples with known CO2 concentrations were used to calibrate the TCD signal. The transient mass balance of the mini column was carried out to estimate the total CO2 captured by the solutions. In a typical procedure, 700 ml of the dust solution was put in contact with a 150 ml/min flow of gas mixture. Samples of the gas mixture were taken every 5 min, and the flow of gas mixture was terminated when the CO2 concentration at the outlet of the column was the same as that at the inlet. As in the titration experiments, CO2 absorption was repeated 2, 3, or 4 times (at 24-h intervals) for some dust solutions until the CO2 concentration at the outlet remained constant after 24 h of aging.
As previously stated, EAF dust comprises Ca, Mg, Na, and K (Table 2), and the oxides of these compounds react easily with water to produce hydroxides. For instance, CaO immediately interacts with wet air, thereby producing Ca (OH)2. Hydroxides (strong alkali electrolytes) can also be dissolved in water to produce metal ions (Ca+2, Mg+2, Na+1 and K+1) and hydroxyl ions (OH–). Other metals high in dust such as Fe and Zn have a tendency to react easily with oxygen to produce the corresponding oxides; these oxides are not soluble in water and do not readily produce hydroxides. However, Fe and Zn oxides could be dissolved in alkaline solutions but their solubility decrease dramatically in neutral solutions. Moreover, complex oxides from Fe and Zn are found in the dust, as was exposed before, and their solubility under alkaline conditions is not yet reported. In this work, dust solutions were prepared, and the pH measurements attest the nature of the solutions. All the solutions were reddish-brown and contained suspended solids. In some cases, the pH was as high as 13, indicating the presence of strong alkalis. Alkaline solutions can be corrosive and cause damage to human health. In industrial practices, EAF dust is sometimes pelletized, placed in open silos for storage, and then disposed of in landfills or sent to metal recovery facilities. Under such situations, the period at which the environment is exposed to dust depends on industrial protocols. If the dust is exposed to rain, there could be a possibility of environmental damage.
Table 1 shows the pH measured from all the fresh dust solutions (pH measured under stirring). The results suggest that EAF dust reacts with water to produce hydroxides, after which OH– ions dissolve, thereby increasing the pH. This phenomenon can be attributed to four metals: Ca, Mg, Na, and K. Table 3 shows the concentrations of these metals and their corresponding OH– concentrations, which were determined under the assumption that CaO, MgO, Na2O, and K2O are found in EAF dust. These components quickly produce hydroxides in the fresh solutions.
| Compound | mol of hydroxides possibly generated from the metal (mmol/kg of dust) | mol ratio OH/M of the corresponding hydroxide | Theoretical OH– ions available (mmol of OH–/kg of dust) | Solubility of corresponding hydroxide in water (mmol of M-(OH)n per liter of water) |
|---|---|---|---|---|
| Ca | 778.4 | 2 | 1556.8 | 24.97 |
| Mg | 1023.2 | 2 | 2046.4 | 0.02 |
| Na | 1600.9 | 1 | 1600.9 | 27750 |
| K | 1049.6 | 1 | 1049.6 | 21210 |
| Total | 6253.7 |
n=1 for Na and K, 2 for Ca and Mg
M=Ca, Mg, Na, or K
From samples 1 to 3 of the fresh solutions showed an increase in pH, but samples 4–7 exhibited a comparable pH even as the dust concentration was the highest in sample 7. This result can be explained by the solubility of hydroxides (Table 3). For instance, sample 1 required 19 ml of water (95 ml of water/5 g of dust) to dissolve the hydroxides and other compounds in 1 g of dust. The OH– concentrations of all the samples was estimated using ASPEN, and the results are shown in Table 1. The table indicates that the OH– concentrations in the fresh solutions (for which pH was measured) were lower than those estimated using ASPEN and the electrolyte NRTL model. This discrepancy may be attributed to the fact that simple hydroxides and other soluble compounds present in the dust dissolve first and reach solubility limits. These phenomena were not observed in the ASPEN estimations because only Ca, Mg, K, and Na as simple hydroxides were considered in the calculations, i.e. oxides and others were not included. In every case, these results suggest that strong basic solutions can be produced from EAF dust even at low dust concentrations. The origin and composition of EAF dust were discussed in previous research,6,7,8) which show that simple and complex oxides are present in dust samples. Simple oxides and their corresponding hydroxides dissolve first until the fresh solutions are saturated. This feature can explain the slight differences between the pH of fresh samples 1–7.
Table 1 shows the concentration of bases (derived by titration with the HCl solution) of each fresh EAF dust solution. The concentrations were obtained by one round of titration for each solution. Conventional titration is useful because the volume of HCl used for neutralization can be converted into the OH– concentration in EAF dust solutions. Conventional acid–base titration is based on acid–base reactions, such as
The results show that the amoung of bases corresponded to the EAF dust concentrations in the solutions. This phenomenon can be explained by the solubility limits of water and the hydroxide concentration in the EAF dust solutions. As previously discussed, the fresh EAF dust solutions dissolved bases until solubility limits were reached, indicating that some bases may be available but undissolved in dust solutions. When the HCl solution was added to the EAF dust solutions, water and metal salts were produced and the dust solutions recovered part of their ability to dissolve bases. These processes can recur until (a) water is saturated but the dissolution of new bases dominates; (b) water is totally saturated; or (c) metal oxides (and their corresponding hydroxides) are consumed.. After 24 h of aging with mixing, some additional bases were dissolved and more rounds of titration were needed. It is possible that during titration, more bases are dissolved until the mass transfer of becomes the dominant reaction. Note, however, that the titration conditions for each solution differed. For instance, four different rounds of HCl titration were required to completely neutralize sample 4, but two rounds were sufficient for sample 1. Results of tritration were used for a roughly estimation of the amoung of CO2 the solution can absorb. Figure 1 compares the total amount of bases derived by titration and the concentration that would be obtained if only NaOH and KOH are dissolved (corresponding to Na and K content; Ca and Mg were disregarded in the estimation because they exhibited the lowest solubility). In all the samples, the total bases concentration estimated by titration was less than that estimated under the NaOH/KOH scenario; 85% (on average) of the hypothetical bases were measured. This expected feature is attributed to the exclusion of more complex oxides in the calculation shown in Fig. 1. For example, highly stable mixed oxides, such as MgFe2O4, could be found in the EAF dust and their contributions were lower than that of the corresponding MgO simple oxide. Moreover, Ca and Mg have low solubility. In such cases, the correlation between NaOH and KOH and the concentration of bases estimated by titration suggests that the high pH of the EAF dust solutions is related to the Na and K content in dust. The aforementioned hypothesis is useful because in industrial practices, only Ca, Mg, Na, and K are easily detected by simple equipment. The results of the study are expected to facilitate the convenient estimation of OH– and bases content in solutions that contain EAF dust.
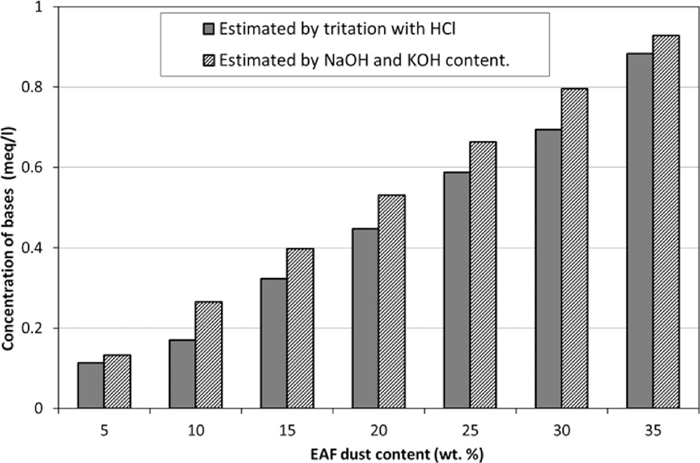
Concentration of bases of the solutions with several dust content.
Finally, CO2 capture was carried out for all the samples (derived from the fresh samples), as described in the experimental section. The conditions of the absorption experiment on all the samples were the same; that is, the final CO2 captured was, on average, 1.52 × 10–3 mol of CO2/g of dust in the solutions (standard deviation, 0.08 × 10–3 in respective units). The differences between the CO2 captured from samples 1–7 were the factors used to determine the manner by which total saturation with CO2 was carried out; that is, the high-dust samples required more rounds of saturation similar to the requirement in the HCl titration experiments. CO2 can be dissolved in water to produce H2CO3, and this weak acid reacts with a base as follows:
The last general reaction shows that each CO2 mole reacted with two hydroxyls to produce a mole of metal carbonate and water. However, it is know that CO2 is absorbed mainly as CO2–2 (carbonate) when pH>10 and mainly as HCO2–1 (bicarbonate) when 7< pH<9; this indicate that the amount of bases does not show linearly with the CO2 absorbed. Usually mole of CO2 used for neutralize is less than mole of HCl required for neutralize alkaline solutions. Therefore, the CO2 captured was in agreetment with this. To illustrate, on the basis of the solubility of CO2 in water (Henry’s law constant=1640 atm at 298 K) and the titration results under assumption that mole of CO2 is the same that mole de HCl used for neutralization, 100 g of sample 4 result in 3.33 × 10–2 mol of CO2 captured (8.1315 × 10–4 mol of CO2 dissolved in 80 g of water + 2.21 × 10–3 mol of CO2/g of dust*15 g of dust=mol of CO2 captured by 100 g of sample 4). The experimental results showed that 100 g of sample 4 captured 2.22 × 10–2 mol of CO2.
3.1. Process of EAF Dust Neutralization by CO2 from EAF (Brief Description)The capacity of EAF dust to adsorb CO2 can be useful in steel plant operations for two reasons: (a) CO2 capture and (b) treatment of EAF dust to decrease the alkaline properties of such dust at the same time that this material is produced. The process is represented in Fig. 2. Under fusion and liquid steel refinement, high-temperature gases (~1873 K) with dust are extracted from an EAF. These components then mix with air to induce reaction with the remaining CO to produce CO2, after which the gas–dust–air mixture is fed into a separation chamber where large particles are separated from the gases and fine particles. Large particles are periodically recovered and introduced to sedimentation equipment. Gases with fine particles are fed into equipment where solutions containing dust are sprayed directly onto the gases; this is the most important step in the process because the spraying solution captures CO2, removes new dust particles, and cools gases. The gases with low dust content are transported to baghouses and the dust solution is recovered. At this stage, metal oxides react with water and CO2, thereby producing carbonates. The solution that contains dust and carbonates is fed into a stirrer tank, to which fresh water and dust are also fed to maintain pH of spray solution. Some of the solution from the stirrer tank is transported to the sedimentation equipment where water is recovered and recirculated, whereas the sediment dust with carbonates but without alkaline properties is sent to disposal.

Simultaneous CO2 capture and dust neutralization.
This process can capture up to 66 kg of CO2/ton of EAF dust. Aside from requiring simple equipment, a number of other advantages are presented by such process: dust from flue gases are pretreated (neutralized), CO2 is captured, and dioxin production may be avoided.
The properties that enable the use of EAF dust in the production of alkali solutions with high pH (~14) were evaluated by the synthesis of solutions with different dust concentrations. The results suggest that metal oxide content (aside from heavy metal content) can be considered in the handling and disposal of EAF dust because metal oxides quickly react with water to produce alkalis.
The solutions used in the study were titrated with HCl and with CO2 to determine the possibility of simultaneous CO2 capture and dust neutralization. The EAF dust solutions captured CO2, thereby demonstrating their potential as materials with a dual purpose: decreasing CO2 emission and neutralizing the alkaline properties of EAF dust solutions.
The process that capitalizes on EAF dust properties was described with a simple diagram of high functionality (Fig. 2). The process also features a step wherein the gases from an EAF are cooled and dust is removed to minimize the possibility of dioxin formation.