2015 Volume 55 Issue 4 Pages 765-774
2015 Volume 55 Issue 4 Pages 765-774
Binderless briquetting of lignite at 100–200°C and subsequent carbonization produces formed coke with tensile strength (ST) of 5–40 MPa, while the briquetting often requires mechanical pressure over 100 MPa. High reactivity is another feature of the lignite-derived coke, and this arises from highly dispersed metallic species such as alkali/alkaline-earth metallic species and ferrous/ferric ones that catalyze CO2 gasification. This work investigated effects of leaching of those metallic species in aqueous solution of hydrochloric acid, acetic acid or oxalic acid on the reactivity and ST of resulting coke from a lignite. The leaching at pH ≤ 1 removed catalytic metallic species near completely, reducing the coke reactivity by a factor of 8–15. The reduced reactivity was similar to the reactivity of coke from a typical coking coal. The leaching at pH ≤ 2.2 increased ST from 6 to 13 MPa for briquetting at 200°C and 32 MPa. The performance of leaching with oxalic acid, of that solution had pH of 0.75 at 1 mol/L, was much better than that with acetic acid. This work also examined another type of leaching, oxidation of the lignite in aqueous solution of hydrogen peroxide, which produced organic mono-/di-acids in-situ from the oxidation of aromatic carbons of the lignite. The degree of reduction of the coke reactivity was between that for leaching at pH of 1 and 2. The degradation of macromolecules enhanced plasticizability of the lignite under briquetting and increased ST of the resulting coke to 22 MPa.
Production of metallurgical coke from lower rank coals such as sub-bituminous coals and lignites is a key technology toward future sustainable steel production. Lignite undergoes neither softening or fusion upon heating unless the heating rate is extremely high.1) A highly porous nature of the lignite tends to inhibit occurrence of fluidity when blended with caking coal.2) The lignite is thus an undesirable feedstock of coke as far as carbonized in conventional ovens.
The present authors proposed production of formed coke from lignite by applying a sequence of binderless hot briquetting and carbonization, and demonstrated preparation of coke with cold tensile strength over 30 MPa.3) Such high strength arose from thermally and mechanically induced plasticization of the organic matrix of lignite, which caused particles’ deformation and coalescence, and then formed highly densified briquette. The briquette was successfully transformed into high-density coke, thanks to no occurrence of either fluidity or swelling. However, briquetting required mechanical pressure as high as 100 MPa or even higher and temperature as high as 200°C. Reduction of briquetting pressure and temperature was left as an important technical subject.
A particular feature of lignite is the presence of metallic species such as alkalis (Na and K), alkaline earths (Ca and Mg) and iron (Fe), major portions of which are associated with carboxylic groups in form of ion-exchanged cations. The content of these metallic species in Victorian lignite (brown coal) is well below 1 wt%-dry,4,5) but these species, which are highly dispersed in the carbonaceous matrix, play significant catalytic roles in gasification of carbonized lignite (char or coke) with CO2 as well as steam.6) In general, the gasification of lignite-derived char is much more rapid than that of chars from coals of bituminous and higher ranks,7) and this is primarily attributed to the catalysis of the above-mentioned metallic species.7,8)
It is easily expected that the reactivity with CO2 of lignite-derived coke is much higher than that of conventional coke. Such higher reactivity may be effective in future operation of blast furnaces with lower temperature in thermal reserve zones.9,10) In fact, there have been a number of studies on the promotion of CO2 gasification of coke by adding catalytic materials such as CaCO3,11,12) other carbonates,12) Fe2O3,11,13) Fe3O413) and iron ore.9,14) The increase in the reactivity (rate of gasification) by adding those particulate catalysts seemed to be at most several times or less even when the catalyst loading was as much as 10 wt% or more.9,10,11,12,13,14) According to Miura et al.,15,16) chars from lignites are gasified at rates higher than those from bituminous coals by factors of even several ten times. Thus, coke from lignite, even if it has sufficiently high strength, may be too reactive to be used in blast furnaces. Reduction or control of the reactivity of the lignite-derive formed coke, which has not been either examined or demonstrated, are therefore essential technical subjects for its effective use.
Removal of metallic species from lignite prior to carbonization is the most straightforward and effective way to control the reactivity of resulting coke. There have been a number of studies on the leaching of metallic species from lignites, but many of those studies employed inorganic acids such as hydrochloric acid (HCl), nitric acid (HNO3) and sulfuric acid (H2SO4).17,18) Use of inorganic acids is impractical because of carryover of Cl, N or S to downstream processes. Application of organic acids, i.e., lower carboxylic acids, to the leaching is free from the above-mentioned problems. However, such organic acids are weak ones and therefore provide limited concentration of proton (H+) that is essential for the leaching of organically bound metallic species. The present authors19) recently investigated preparation of formed coke from some different biomass resources that had been heat-treated under hydrothermal conditions (i.e., in hot and compressed water at 200–300°C). The hydrothermal treatments decomposed carbohydrates converting them partly to lower organic acids while leaving lignin with slight depolymerization or polymerization. The formed acids reduced pH of the aqueous phase to ca. 3, thereby leaching Na, K, Ca and Mg effectively. The hydrothermal treatment was, however, ineffective for leaching of metallic species from lignite due to very limited formation of organic acid. This was confirmed by the present authors’ investigation preliminary to this work.
The primary purpose of this work was to investigate the leaching of metallic species from a well-known type of lignite with mono- and di-organic acids, and examine its effects on the reactivity and strength of resulting coke as well as briquettability of the lignite. This work also investigated treatments of the lignite in aqueous solution of hydrogen peroxide (H2O2), which oxidatively depolymerized the lignite in-situ forming lower organic acids that played roles of leaching agents.20,21)
A Victorian lignite (Loy Yang) and a typical coking coal (coal A) were used as the starting samples. The lignite was pulverized to sizes smaller than 106 μm, dried under vacuum at 60°C until the moisture content decreased to around 10 wt% on a wet basis. The coal A was subjected to only the pulverization in the same way as the lignite. The lignite and coal samples were stored in a plastic bottle at ambient temperature until use. The elemental compositions and ash contents of the lignite and coal A are available in Table 1. The contents of metallic species in the lignite were as follows: Na; 0.079, K; 0.012, Mg; 0.062, Ca; 0.058, Fe; 0.067 wt% on a dry basis.
| Lignite/coal | C | H | N | O + Sa | ash |
|---|---|---|---|---|---|
| wt%-dafb | wt%-dbc | ||||
| Lignite | 66.9 | 4.8 | 0.6 | 27.0 | 0.8 |
| Coal A | 87.0 | 4.9 | 2.0 | 5.6 | 8.0 |
a) By difference. b) On dry and ash free basis. c) On dry basis.
Leaching of metallic species of the lignite was performed in five different aqueous solutions of hydrogen chloride (HCl) at concentration of 3 mol L–1, that at 0.1 mol L–1, that at 0.01 mol L–1, acetic acid (AcA) at 1.0 mol L–1, and oxalic acid (OxA) at 1.0 mol L–1. All of the acids were in special reagent grades. More detailed conditions of the leaching are shown in Table 2. The time period and temperature for every leaching were 24 h and 60°C, respectively. After the leaching, the solid (i.e., treated lignite) was recovered by a sequence of filtration, exhaustive washing with deionized water (electric resistance ≈ 18.2 MΩ), and drying under vacuum in the same way as mentioned in the previous section. The leaching treatments with the solutions of 3 mol L–1 HCl, 0.1 mol L–1 HCl, 0.01 mol L–1 HCl, AcA and OxA will be denoted by HCl-pH-0.5, HCl-pH1.0, HCl-pH2.0, AcA and OxA, respectively. The initial pH of the solutions of OxA and AcA were in good agreement with those in literature.22) Change in the mass of the lignite was within ± 1 wt% on the dry basis regardless of the type of acid used. This was due to insignificant extraction of organic matter into acidic aqueous phase and also complete elimination of the acid.
| Treatment | acid | concentration mol L–1 | mass of solution, g | mass of lignite g-dry | initial pH – | final pH – |
|---|---|---|---|---|---|---|
| HCl-pH2.0 | HCl | 0.01 | 30 | 4.5 | 2.00 | 2.00 |
| HCl-pH1.0 | HCl | 0.10 | 30 | 4.5 | 1.00 | 1.00 |
| HCl-pH-0.5 | HCl | 3.00 | 30 | 4.5 | –0.48 | –0.48 |
| AcA | acetic acid | 1.00 | 30 | 4.5 | 2.16 | 2.12 |
| OxA | oxalic acid | 1.00 | 30 | 4.5 | 0.75 | 0.76 |
| HPO-0.20 | H2O2 | 0.88 | 30 | 4.5 | 6.01 | 2.15 |
| HPO-0.38 | H2O2 | 1.76 | 30 | 4.7 | 6.03 | 1.79 |
All of the treatments were performed at 60°C and for 24 h.
The leaching was also performed separately from the above-mentioned series with repeated use of spent solutions of OxA at a concentration of 1 mol L–1. After the leaching (first run), the spent solution was recovered by the filtration with a recovery of 80–85% in mass. Such limited recovery was due to the retention of the solution by the porous lignite. A makeup solution of OxA at 1 mol L–1 was then added to the spent solution so that the mass of the solution was the same as that for the first run. The solution of OxA thus prepared was used for the second run. The third and fourth runs were carried out with the same procedures as the second run.
2.3. Oxidative Leaching with Hydrogen PeroxideThe lignite was treated in a solution of hydrogen peroxide (HPO) at a concentration of 0.88 or 1.76 mol-HPO L–1. These concentrations corresponded to HPO/lignite mass ratios of 0.20 and 0.38, respectively. The treatments are then denoted by HPO-0.20 and HPO-0.38 in Table 2. Each solution was prepared by mixing commercially available aqueous solution (30 wt%) and deionized water. The concentration of HPO was determined by a general redox titration. The time period and temperature for the treatment were 24 h and 60°C, respectively, according to literature.20,21) The oxidized lignite was isolated from the solution by filtration and subsequent washing with deionized water. The conversion of HPO was determined by quantifying HPO in the spent solution. The conversion was 88–92% regardless of the initial HPO concentration. The mass yields of the oxidized lignite were 95.0 and 93.8 wt% on the dry basis for HPO-0.20 and HPO-0.38, respectively.
2.4. Quantification of Metallic Species in LigniteThe starting lignite and treated ones were subjected to ashing by applying a temperature programmed combustion method.23) The resulting ash was digested in a mixture of aqueous solutions of hydrogen fluoride and nitric acid, resolidified by evaporation of the water and acids, and then redissolved into an aqueous solution of methane sulfonic acid. The solution was analyzed by ion chromatography for quantification of Na, K, Mg and Ca. More details of the procedure are available in literature.24,25) Fe in the ash was quantified by a general sequence of dissolution and colorimetry.
2.5. Preparation of CokeThe lignite and treated ones were briquetted at temperature (Tb) of 130 or 200°C and mechanical pressure (Pb) of 32 or 128 MPa. The briquettes were in form of disc with diameter and thickness of ca. 14 mm and 5 mm, respectively. Details of the procedure were reported elsewhere.3,19,26) The briquettes were carbonized by heating under continuous flow of atmospheric nitrogen (N2) with a heating rate, peak temperature and holding time of 3°C min–1, 1000°C, and 10 min, respectively. The coal A was carbonized according to the same procedure of heating as above but without briquetting.
2.6. Gasification of Char and CokeReactivities of coke and char samples with CO2 were investigated. The char reactivity was analyzed according to a previously reported method.27,28) The starting lignite, treated lignite or coal A (ca. 5 mg) was heated in a thermogravimetric analyzer (TGA) under continuous and atmospheric flow of N2 (700 ml-stp min–1) at a heating rate of 10°C min–1 up to 905±1°C. Then, the gas flow was switched to that of a equimolar mixture of N2 and carbon dioxide (CO2) at the same total volumetric flow rate as above while the temperature was maintained at 905±1°C. The gasification of the char was thus performed in-situ. The temperature for the gasification, which was lower than that for the coke preparation, was limited by the furnace performance. The conversion of char by CO2 gasification, X, was defined by the following equation.
| (1) |
mC and mC0 are the mass of the carbonaceous part of char at a given time (t) and that at t = 0, respectively. t = 0 was defined as the time when the mass of char started to decrease by the gasification. The mass fractions of the carbonaceous and ash parts of the char were determined by means of combustion. The CO2 gasification of coke samples was performed at the same temperature and atmosphere as above. A specimen of coke was crushed into pieces, and one of them with a mass of around 5 mg was subjected to the gasification.
2.7. Characterization of CokeTensile strength (TS) of coke was measured at ambient temperature. The procedure was reported elsewhere.29) Four to six specimens were subjected to the tests for every type of coke. TS was determined from the stress at the breakage and dimension (diameter and thickness) of the specimen in form of disc.28) The standard deviation for the measured TS was within 12% of the average for all of the types of coke. The Brunauer–Emmett–Teller (BET) and CO2 surface areas were measured for two selected char samples. Details of the measurements were reported previously.30)
This section reports and discusses the characteristics of the leaching, gasification of char, that of coke and its properties in sequence, and demonstrates simultaneous modification of the reactivity of coke and its strength by leaching with the organic acids.
3.1. LeachingFigure 1 shows the content of each metallic species in the lignite as a function of pH of the aqueous solution at the end of leaching (see Table 2). pH is a factor most responsible for the removal of each metallic species, in other words, extent of exchange between metallic cations and protons.31,32) The solid line shows the effect of pH on the content for the HCl treatments.

Residual Na, K, Mg, Ca or Fe content of the lignite as a function of pH of the aqueous solution.
Na is the most abundant in the original lignite on a molar basis, but its content decreases greatly regardless of pH. The content of K, which is much less abundant than Na, decreases more extensively by the leaching with the organic acids (AcA and OxA) than HCl. Though not clearly seen in the figure, a similar trend was confirmed for the Na content. The organic acids thus removed the alkalis more effectively than HCl at equivalent pH. This is explained by more affinity with the organic matrix of the organic acids than HCl, which is probably too hydrophilic to penetrate into the entire part of the organic matrix of the lignite.23) The removal of Ca, Mg and Fe depends more strongly on pH than that of Na and K. Such a trend is in broad agreement with previous reports,33) and attributed to divalent nature of Ca and Mg ions, and divalent or trivalent nature of Fe ions. In general, stability of complexes of organic acid and metallic cation increases with the valence of the latter, while dissociation of them requires lower pH. The use of organic acids is also more effective than that of HCl in the removal of Mg and Ca. The trend for the Fe removal is similar to those of Ca and Mg.
The HPO oxidation of the lignite produced lower mono- and di-acids such as oxalic, formic, acetic, glycolic, malonic and succinic acids, thereby decreasing pH of the aqueous solution to 1.8–2.1. The removal rates of all the metallic species by the leaching with HPO are, nonetheless, lower than those with AcA and OxA. This is explained qualitatively by introduction of carboxylic groups in the lignite (solid phase) while formation of acids and their release to the aqueous phase.20,21) Carboxylic groups in the solid phase competed with the organic acid in the aqueous phase toward complexation with metallic cations. The increased concentration of carboxylic groups in the solid made the leaching more difficult.
3.2. Reactivity of Char with CO2Chars from the original and treated lignites were subjected to the in-situ CO2 gasification. Figure 2 shows change in the unconverted fraction of char, 1 – X, with t. Three indices; R0.25, R0.50 and R0.75, are here introduced for evaluating the char reactivities at different conversions of 0.25, 0.50 and 0.75, respectively. These indices are average rate of gasification (i.e., dX/dt) around X = 0.25, 0.50 or 0.75, which are given by the following equations.
| (2), |
| (3), |
| (4) |

Time-dependent changes in the unconverted fraction of char during in-situ CO2 gasification. Temperature; 905±1°C, Partial pressure of CO2; 0.05 MPa. Note that different time scale are employed in the right and left graphs.
ta is defined as the time required for the char conversion up to X = a. Table 3 summarizes the char reactivities.
| Treatmemt ID | R0.25 | R0.50 10–2 min–1 | R0.75 |
|---|---|---|---|
| Original | 5.34 | 4.49 | 3.72 |
| HCl-pH2.0 | 0.995 | 0.896 | 0.588 |
| HCl-pH1.0 | 0.431 | 0.313 | 0.198 |
| HCl-pH-0.5 | 0.439 | 0.307 | 0.173 |
| AcA | 1.52 | 1.19 | 0.655 |
| OxA | 0.393 | 0.279 | 0.162 |
| HPO-0.20 | 2.46 | 1.96 | 1.24 |
| HPO-0.38 | 1.65 | 1.466 | 0.985 |
| Coal A | 0.236 | 0.169 | 0.092 |
The char from the original lignite undergoes much more rapid gasification than that from the coal A with R0.25, R0.50 and R0.75 greater by factors of 20–40. Such high reactivity is assigned primarily to the catalysis of the metallic species. The reactivity decreases as the removal of the metallic species becomes more significant. The gasification of the chars from HCl-pH1.0, HCl-pH-0.5 and OxA-treated lignites is still faster than that from the coal A, but R0.25, R0.50 and R0.75 for the formers are twice or less of those for the latter. Thus, the leaching at pH ≤ 1 reduced the reactivity of char from the lignite by a factor of 12–23. The char reactivity can be modified over a wide range by controlling pH of the aqueous solution for the leaching. pH of the solution can easily be controlled by the concentration of acid regardless of type.
The catalysis of the metallic species is further discussed. Figure 3 plots R0.50 against the contents of metallic species in the original or treated lignites. Though not shown in the figure, very similar relationships with contents of metallic species were confirmed for R0.25 and R0.75. The solid lines are drawn for indicating the trend of R0.5 that increases with increasing content of metallic species in a linear or semi-linear manner. The chain line is drawn assuming the catalysis of Na and K, which is agreed generally for the gasification of carbonized solids. The horizontal broken line in each graph indicates R0.50 of the chars from HCl-pH1.0, HCl-pH-0.5, and OxA-treated lignites, which are near equivalent with each other (see Table 3). As seen in Fig. 1, there are some varieties in the residual contents of metallic species among the HCl-pH1.0, HCl-pH-0.5 and OxA treatments. The metallic species left after these treatments, if any, had no or very little catalytic activities in the CO2 gasification. This is reasonably explained by reactions of the metallic species with Si and Al species, in other words, deactivation of the metallic species, during the pyrolysis and/or subsequent gasification. Na, K, Ca, Mg and Fe easily react irreversibly with SiO2 and Al2O3 to form silicate, aluminate or aluminosilicates at elevated temperature.35,36) The total amounts of Na, K, Ca, Mg and Fe in the HCl-pH1.0, HCl-pH-0.5 and OxA-treated lignites were 6, 14 and 7 mmol kg-dry–1, and these were sufficiently smaller than those of Si and Al, which were ca. 70 and 90 mmol kg-dry–1, respectively. Assuming complete or near complete deactivation of the metallic species during the pyrolysis or gasification, the gasification of the chars from the lignites after leaching at pH ≤ 1 was practically non-catalytic.
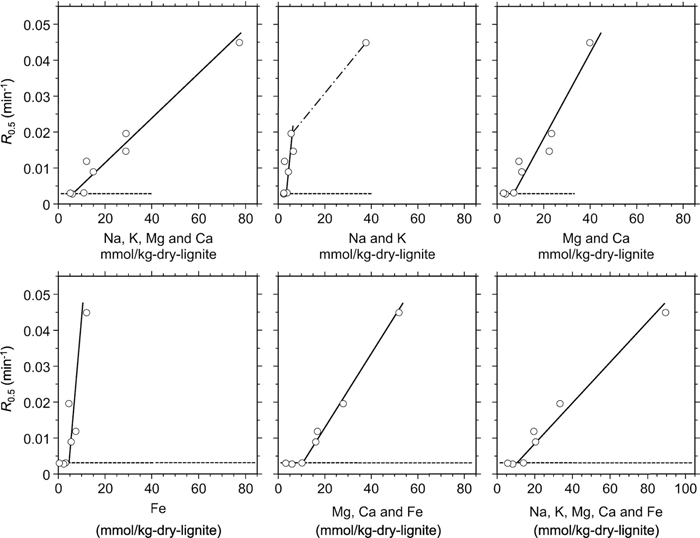
Relationship between R0.5 and content of metallic species.
R0.5 increases with the total content of the five metallic species as well as those of Ca+Mg and Fe, if the content is greater than ca. 10 mmol kg-dry–1. There is a particular trend with the relationship between the Na+K content and R0.5. It appears at Na+K content of 2–6 mmol kg-dry–1 that only a small decrease in the content causes significant decrease in R0.5. This does not mean high activity of Na and K at such content. It is rather interpreted that neither of Na or K is active at 2–6 mmol kg-dry–1 and that change in R0.5 is caused by that in the contents of Ca, Mg and Fe. On the other hand, in the range of Na+K content over 6 mmol kg-dry–1, these alkalis must catalyze the gasification, according to previous studies.4,5,15,16) It can thus be said that all of the five metallic species play catalytic roles during the gasification of char from the original lignite while Ca, Mg and Fe are the major catalysts in the gasification of chars from the treated lignites. The catalytic activity of Mg was often suspected in previous studies on the gasification of coal char,37,38,39) but it is difficult to deny its activity. Though not shown in Fig. 3, R50 was correlated well with the individual contents of Mg and Ca. Hippo and Walker Jr.40) investigated CO2 gasification of chars from various types of coals, and found the char reactivity increased with Mg content unless it exceeded ca. 80 mmol kg-char–1.
As mentioned previously, it is believed that the chars from the HCl-pH1.0, HCl-pH-0.5 and OxA-treated lignites undergo non-catalytic gasification. Even if so, the reactivities of those chars are still higher than the char from the coal A. At least a part of such difference is ascribed to that in the porous structure. The chars were prepared from the coal A and HCl-pH-0.5 treated lignites by heating them to 900°C with the same temperature histories as for the gasification, and the resulting chars were subjected to the surface area measurements. The char from the treated lignite had BET and CO2 surface areas of 410 and 740 m2 g–1, respectively, and were, as expected, much greater than those of the char from the coal A, which were only ca. 3 and 100 m2 g–1, respectively. It was recently reported that non-catalytic steam gasification of char from Loy Yang lignite occurred at a rate independent of both the BET and CO2 surface areas.30) The present CO2 gasification is, however, not the case. The mechanism of CO2 gasification will be discussed in brief later.
3.3. Reactivity of Coke with CO2Figure 4 compares the profiles of coke gasification with that of char for the individual lignites. The rate analyses revealed that the rate of coke gasification was lower than that of corresponding char by a factor of around 1.7 over the range of conversion, except the cokes and chars from the HPO-treated lignites. Slower gasification of coke than char is primarily due to higher carbonization temperature for coke (1000°C) than that for char (900°C). The shapes of the conversion curves for the coke and char were very similar to each other, and this indicated similarity in the characteristics of gasification such as relative contributions of the catalytic gasification and non-catalytic one. For the HPO-treated lignites, the gasification of coke is slower than that of the corresponding char by 3–4 times, and also slower than that from the AcA-treated lignite. It is seen in Fig. 2 that the chars from the HPO-treated lignites are gasified more quickly than that from the AcA-treated one. Thus, Fig. 4, taken together with Fig. 2, shows that the correlation between the char reactivity and abundance of metallic species is not necessarily valid for the coke reactivity when the cokes from the HPO-treated lignites are considered. This is attributed to a particular type of carbon structure of the coke from the HPO-treated lignite, which would be different from that of the corresponding char, but no clear explanation is available at present.

Time-dependent changes in (1–X) of coke during CO2 gasification and its comparison with those of char gasification. The cokes were formed by carbonization of briquette that had been prepared with Pb and Tb of 32 MPa and 200°C, respectively.
Figure 5 compares the conversion profiles for the cokes from the HCl-pH-0.5, HCl-pH-1.0 and OxA-treated lignites, which are almost identical with one another. This is consistent with non-catalytic nature of the gasification of not only the chars but also cokes from the lignites treated at pH ≤ 1. The kinetics of coke gasification was analyzed by a random pore model (RPM)41) that has often been applied to kinetic analysis of char gasification. The RPM presents the kinetics by
| (5) |

Conversion profiles for cokes from HCl-pH-0.5, HCl-pH1.0 and OxA-treated lignites. The cokes were formed by carbonization of briquette that were prepared with Pb and Tb of 32 MPa and 200°C, respectively.

Comparison of conversion profiles for cokes from coal A and HCl-pH-0.5 treated lignite. The coke from HCl-pH-0.5 treated lignite was formed by carbonization of briquette that were prepared with Pb and Tb of 32 MPa and 200°C, respectively.
More importantly, Fig. 6 demonstrates that the reactivity of the coke from the treated lignite is very similar to that from the coal A (see Table 4). The ratio of k0 for the coke from the lignite to that from the coal A is as small as 2, which is attributed to the difference in the reactivity and concentration of active site, in other words, chemical nature of carbonaceous part of coke. The leaching of metallic species prior to briquetting and carbonization thus enables to reduce the reactivity of resulting coke to a level similar to that of coke from coking coal.
| Treatment or sample ID | R0.25 | R0.50 10–2 min–1 | R0.75 |
|---|---|---|---|
| Original | 1.84 | 1.88 | 1.77 |
| HCl-pH-0.5 | 0.228 | 0.183 | 0.112 |
| Coal A | 0.185 | 0.161 | 0.108 |
Figure 7 shows the yields and properties of cokes from the original and treated lignites. The coke yield is independent of the extent of leaching of metallic species, but decreases due to the treatments with HPO. The liquid-phase oxidation introduces oxygen functionalities, which decompose to light gases such as CO2, CO and H2O reducing the coke yield.42)

Yields and properties of cokes. Pb and Tb are 32 MPa and 200°C, respectively.
The treatments with HCl, AcA and OxA increase TS of resulting coke from 6 to 13 MPa, while the degree of elimination of metallic species is not a determining factor of TS. The total content of the five metallic species in the original lignite was ca. 90 mmol kg-dry–1, and it was reduced largely to 6–20 by the leaching with HCl, AcA and OxA. The metallic species played roles of cross-links in the macromolecular network of the lignite.43,44) Removal of the metallic species could therefore enhance flexibility and plasticizability of the organic matrix of the lignite under mechanical pressure. However, the enhanced plasticizability is not necessarily supported by the present data, because the bulk densities of the briquettes from the HCl, AcA and OxA-treated lignites are equivalent to that from the original one. In general, organically bound alkali and alkaline earth metallic species suppress the net depolymerization of the macromolecules of lignite, and then the formation of tar and its temporary accumulation in the pyrolyzing solid.45,46,47) Promotion of the net depolymerization leads to formation of more tar and other fusible components, a portion of which can fill small gaps (interstitial spaces) among contactless particles in the pyrolyzing briquette.48) Local softening or fusion inside the briquette is also a potential event during the pyrolysis of the treated lignite, and it helps particles’ coalescence.
It is also noted in Fig. 7 that the HPO treatment increases the briquette/coke densities, and then TS of coke up to 22 MPa. This is ascribed to the oxidative depolymerization45) that enhanced the plasticizability of the lignite, particles’ coalescence, causing densification of the briquette and then that of the coke. The increased briquette density might partly be due to increase in the oxygen content of the lignite by the oxidation. It is, however, not a reasonable explanation of the increased density of the coke, because the oxygen content of the briquette was not a factor for that of the resulting coke any more. Table 5 compares TS among the cokes from the original, HCl-treated and HPO-0.20-treated lignites for different combinations of briquetting pressure and temperature. For the briquetting at 130°C and 32 MPa, TS of the coke from the HPO-0.2-treated lignite is about 5 times that from the original one while slightly higher than that from the HCl-treated lignites. Further increase in the briquetting temperature to 200°C causes an advantage of the HPO treatment over the HCl one, and thus the oxidative depolymerization is more effective than removal of metallic cations for inducing its thermal mobility, i.e., plasticizability of the lignite. On the other hand, when both briquetting temperature and pressure are sufficiently high as 200°C and 128 MPa, respectively, even the original lignite seems to undergo developed plasticization as extensively as the treated lignites.
| Treatment | Pb, MPa | Tb, °C | TS, MPa |
|---|---|---|---|
| Original | 32 | 130 | 2.7 |
| Original | 32 | 200 | 6.3 |
| Original | 128 | 130 | 29.7 |
| Original | 128 | 200 | 31.9 |
| HCl-pH2.0 | 32 | 130 | 12.5 |
| HCl-pH2.0 | 32 | 200 | 13.4 |
| HCl-pH1.0 | 32 | 130 | 12.8 |
| HCl-pH1.0 | 32 | 200 | 13.0 |
| HPO-0.20 | 32 | 130 | 13.7 |
| HPO-0.20 | 32 | 200 | 17.0 |
| HPO-0.20 | 128 | 130 | 33.5 |
| HPO-0.20 | 128 | 200 | 34.0 |
The present authors previously reported linear relationship between the bulk density and TS of coke from the same lignite as used in the present study. Such a relationship arises from that stress concentration occurs at pores with sizes of and over micrometer scale inside the coke.3) Figure 8 demonstrates such a linear relationship for the cokes from the treated lignites. It is noted that the coke from the original lignite is away of the linear relationship. This demonstrates an efficacy of the leaching, and also suggests that a property other than the bulk densities of briquette and coke influences TS. As mentioned previously, metallic cations play roles of cross-links in the macromolecular network of the lignite. It is therefore reasonable that the treated lignites underwent slightly more extensive plasticization eliminating particles’ interfaces, the frequency of which did not necessarily affected the bulk density of briquette. Enhanced tar evolution and more significant depolymerization of the macromolecular network, which were caused by the removal of the metallic species,46) presumably promoted coalescence of particles in mutual contact during the carbonization. The extent of such coalescence would not necessarily influence the bulk density of coke.

Relationship between TS and bulk density of coke from the lignite. Closed and open keys indicate the coke from the original and treated lignites, respectively. Pb and Tb are 32 MPa and 200°C, respectively.
Repeated use of spent solution of acid is necessary for reducing and minimizing the net consumption of acid that is undoubtedly more expensive than lignite. Table 6 lists the conditions and result (only pH of the solution at the end of leaching) of the first to fourth runs of leaching. OxA was chosen as the leaching agent because of its better performance than AcA. The cumulative mass of OxA per that of the lignite decreases from 0.62 to 0.23 within the range up to the fourth run while pH of the spent solution (i.e., that of the solution to be recycled) is maintained around 0.75. Further reduction of the cumulative mass of OxA is hence expected with maintenance of pH or its minimized increase after more repeated runs. Though not demonstrated experimentally yet, the net consumption of OxA for the leaching is simulated numerically. Here are assumed the solution/lignite mass ratio = 6.7 L/kg-dry (average of those for the four runs), the recovery (recycle) ratio of solution = 0.85 (fourth run), and the concentration of OxA = 1.0 mol/L = 0.09 kg-OxA/L. The cumulative loss of OxA per that of the treated lignite is calculated as 0.09 kg-OxA/kg-dry-lignite after a sufficient number of repletion, i.e., at steady state. The increase in the concentration of the metallic species, if the performance of leaching is maintained, is theoretically given as 6.7 times (= inverse of the recovery ratio), and then the total concentration is estimated as ca. 0.13 mol-eq./L, according to the data shown in Fig. 1. The total concentration of the metallic species in the OxA solution is estimated in this way, although it was not measured for the second to fourth runs. It is also estimated that the total concentration had already increased by a factor of ca. 3.2 at the fourth run, while pH of the solution was practically the same as that for the first run. Experimental demonstration of the maintenance of the leaching performance is a technical subject left for the future study, but it is expected that the leaching power of the OxA solution is maintained steady under repeated use.
| Run ID | 1st | 2nd | 3rd | 4th |
|---|---|---|---|---|
| Mass of dry lignite, g | 4.38 | 4.48 | 4.48 | 4.57 |
| Mass of OxA in initial solution, g | 2.70 | – | – | – |
| Mass of OxA in reused solution, g | – | 2.14 | 2.26 | 2.31 |
| Mass of OxA in makeup solution, g | – | 0.56 | 0.44 | 0.39 |
| Total amt. of OxA used for leaching, g | 2.70 | 2.70 | 2.70 | 2.70 |
| Initial pH, – | 0.73 | 0.74 | 0.77 | 0.76 |
| Final pH, – | 0.74 | 0.75 | 0.76 | 0.75 |
| Cummulative mass of OxA per that of lignite, – | 0.62 | 0.37 | 0.28 | 0.23 |
The following conclusions have been drawn in the present study within the ranges of the experimental conditions.
(1) The leaching treatment of the lignite in aqueous solution with pH ≤ 1 removes catalytic metallic species (Na, K, Ca, Mg and Fe) completely or near completely. Use of OxA enables to prepare aqueous solutions with pH well below 1.
(2) The chars and cokes from the OxA-treated lignite and HCl (pH ≤ 1)-treated ones undergo non-catalytic CO2 gasification with rates lower that those from the original lignite by an order of magnitude or even more, and also with rates similar to those from the coal A, a typical coking coal.
(3) The reactivity of coke from the lignite can be controlled by the acid washing over a range of 1–16 times that from the coal A.
(4) Leaching of metallic species at pH < 2.2, causes TS of the resulting coke increase from 6 to 13 MPa for the briquetting at 200°C and 32 MPa.
(5) Oxidation of the lignite in aqueous solutions of HPO produces lower organic acids decreasing pH of the solution to 1.8–2.2, introduces carboxylic groups into the lignite, and depolymerizes macromolecules. The removal of metallic species is less significant compared with that by the OxA and AcA treatments at equivalent pH. The depolymerization enhances plasticizability of the lignite in the briquetting leading to increase in TS from 6 up to 22 MPa for the briquetting at 200°C and 32 MPa.
A part of this work was supported by Japan Society for The Promotion of Science (JSPS) for Grant-in-Aid for Scientific Research A (Grant Number 26249120).