2015 Volume 55 Issue 6 Pages 1206-1209
2015 Volume 55 Issue 6 Pages 1206-1209
Generally, reactions and forming phases during ironmaking can be thermodynamically predicted using equilibrium phase diagram. However, at low temperature it will likely to be different from predicted phases and deviate from equilibrium. Hence, knowledge of solid state reaction at low temperature is required to control the melting behavior of slag phase in blast furnace. Reactions between iron oxide and gangue minerals in ore under at 1373 K under Ar atmosphere were investigated in present work.
Suppression of CO2 discharged from iron- and steel-making companies is an example of the biggest issues for the protection of global environment and sustainable growth of steelmaking industry. One of the ideas to decrease CO2 emission and energy consumption from ironmaking process is to decrease the average process temperature during ironmaking. If it is possible to produce iron at lower temperature, reaction during the process may differ from the present process. Generally, reactions and forming phases during ironmaking can be thermodynamically predicted using equilibrium phase diagram. However, at low temperature the phases will likely to be different from predicted phase and deviate from equilibrium. Raw materials charged into blast furnace includes various minerals or crystal phases. Behavior of slag formation can’t be estimated without considering the initial form, type of crystal phase and reaction between solid minerals and solid iron oxides at low temperature. Hence, knowledge of solid state reaction at low temperature is required. Fundamental reactivity between iron oxide and gangue minerals in ore at 1373 K under Ar atmosphere were investigated in present work.
Samples were prepared by the following procedures. FeO was made by heating reagent grade Fe2O3 at 1200 K for 2 hours under CO2–50 COvol% gas. Fe3O4 was made by heating reagent grade Fe2O3 at 1000 K for 1 hours under CO2–20 COvol% gas. CaO was made by heating reagent grade CaCO3 at 1100 K for 4 hours under air. Also, reagent grade Fe2O3, SiO2, Al2O3, MgO was used for experiments. Average particle size for FeO, Fe3O4, Fe2O3, CaO, SiO2, Al2O3 and MgO was 17 μm, 17 μm, 20 μm, 15 μm, 40 μm, 45 μm and 15 μm, respectively. Oxides were mixed at same weight ratio and weighed 1 g was pressed into ϕ10 mm tablet shape. Oxide tablet was placed on Al2O3 boat and the sample was placed in a horizontal silica tube (O.D. 25 mm, I.D. 21 mm, L. 900 mm)) inside an electric resistance furnace. The sample temperature was heated at 1373 K under Ar flow of 0.05 L/min for 60 min. The purity of Ar was 99.9999% and was dehydrated by molecular sieve during experiment. Then the sample was quenched by pulling out the horizontal silica tube from the furnace. The sample was analyzed by XRD (D8 ADVANCE BRUKER) and semi-quantitative analysis using RIR (Reference Intensity Ratio) method was conducted by PDXL2 software.
To clarify the reactivity between two oxides, 10 samples were prepared. Mixed samples of CaO+FeO, CaO+Fe3O4 and CaO+Fe2O3 was investigated to see the reactivity of CaO and Fe oxide mixture. Also, CaO was mixed with SiO2 or Al2O3 to observe the reactivity between CaO and gangue minerals. To see the reactivity of FeO and gangue minerals, mixed samples of FeO+SiO2, FeO+Al2O3 and FeO+MgO were prepared. Mixed samples of SiO2+Al2O3 and MgO+Al2O3 were also investigated.
Additionally, mixed sample of CaO+FeO+SiO2 was prepared to observe the reactivity between three oxides. Also, CaO+FeO+SiO2+Al2O3 and CaO+FeO+SiO2+MgO mixtures were investigated to clarify the effect of Al2O3 and MgO addition.
Figure 1 shows the XRD pattern of experiment using CaO and SiO2. From CaO–SiO2 binary phase diagram1) shown in Fig. 2, the equilibrium phases that exist at 1373 K for the mixed ratio of present work are CaO · SiO2 and 3CaO · SiO2. However, CaO, SiO2 and 2CaO · SiO2 were observed after the experiment. From the fact that very stable 2CaO · SiO2 compound exists in CaO–SiO2 binary system,1) affinity between CaO and SiO2 is large. However, CaO+SiO2 mixture will not be in equilibrium state after 1 hour heating at 1373 K.

XRD results for CaO+SiO2 mixture heated at 1373 K under Ar for 1 hour.
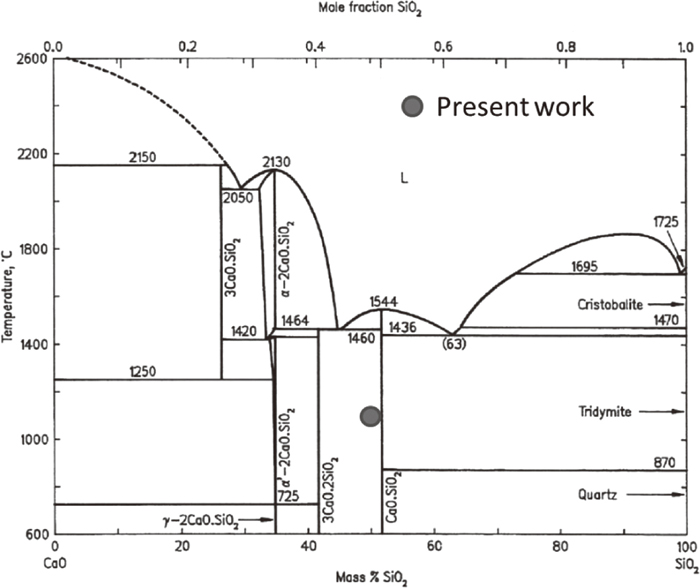
Phase diagram of CaO–SiO2 binary system.1)
Figure 3 shows the phase diagram of CaO–FeO and CaO–Fe2O3 binary system.2) When oxides were mixed at same weight ratio, equilibrium phase that exist at 1373 K are CaO and 2CaO · Fe2O3. Liquid phase exists at 1373 K in CaO–FeO phase diagram, while no liquid phase exist at the same temperature for CaO–Fe2O3 phase diagram. After mixed samples of CaO+FeO was heated at 1373 K, sample was almost completely melted. Partial melting was observed for CaO+Fe3O4 mixture and no liquid phase was observed for CaO+Fe2O3 mixture. It was made clear that liquid formation of CaO and Fe oxide start at low temperature when Fe2O3 is reduced to Fe3O4 and liquid phase increases when further reduced to FeO. The observed phase by XRD for CaO+FeO, CaO+Fe3O4 and CaO+Fe2O3 mixed samples are shown in Fig. 4. The phases observed after experiments were CaO and 2CaO · Fe2O3 and this agree with predicted phases at 1373 K from phase diagram. Peaks for CaO+FeO mixture were weak due to existence of liquid phase. Also, Fe was detected in CaO+FeO and CaO+Fe3O4 mixed sample. It can be said that reactivity of CaO and Fe oxide is large.
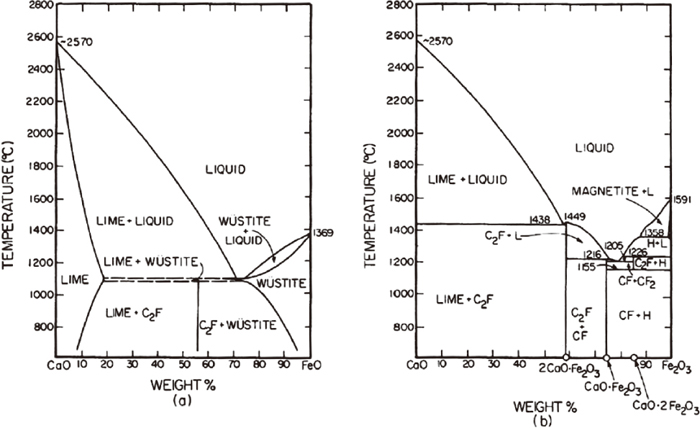
Phase diagram of CaO–FeO and CaO–Fe2O3 binary system.2)
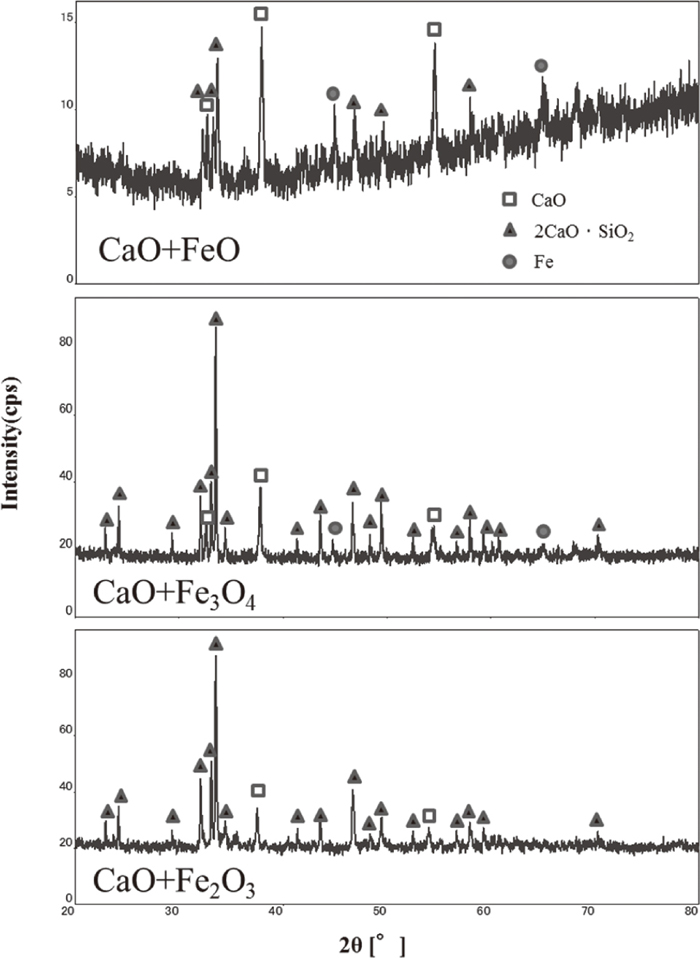
XRD results for CaO+FeO, CaO+Fe3O4 and CaO+Fe2O3 mixture heated at 1373 K under Ar for 1 hour.
Reactivity between two oxides was investigated and it was summarized in Table 1. Also, the XRD patterns of experiment using CaO+Al2O3, FeO+SiO2, FeO+Al2O3, FeO+MgO, SiO2+Al2O3 and MgO+Al2O3 are shown in Fig. 5. Analysis by SEM-EDS was conducted for FeO+MgO mixture and it was confirmed that (Fe, Mg)O was present after the experiment. The reactivity of oxide mixtures that predicted phases agreed with observed phases was considered high. The reactivity of mixtures that didn’t match with predicted phase was considered as moderate. For the mixture of SiO2 and Al2O3, oxide compound was not detected after experiment and the reactivity was low. Reactivity of Fe oxide and CaO tends to be high, while that of Al2O3 is low.
| Mixed oxides | Predicted equilibrium phases | Observed phases | Reactivity | |||||
|---|---|---|---|---|---|---|---|---|
| CaO | Fe2O3 | CaO | 2CaO·Fe2O3 | CaO | 2CaO·Fe2O3 | high | ||
| CaO | Fe3O4 | CaO | 2CaO·Fe2O3 | CaO | 2CaO·Fe2O3 | Fe | high | |
| CaO | FeO | CaO | FeO | CaO | 2CaO·Fe2O3 | Fe | high | |
| CaO | SiO2 | CaO·SiO2 | 3CaO·SiO2 | CaO | SiO2 | 2CaO·SiO2 | moderate | |
| CaO | Al2O3 | 3CaO·Al2O3 | 12CaO·7Al2O3 | CaO | Al2O3 | 3CaO·Al2O3 | CaO·Al2O3 | moderate |
| FeO | SiO2 | SiO2 | FeO·SiO2 | SiO2 | FeO·SiO2 | high | ||
| FeO | Al2O3 | FeO | FeO·Al2O3 | FeO | FeO·Al2O3 | Al2O3 | moderate | |
| FeO | MgO | (Fe,Mg)O | (Fe,Mg)O | high | ||||
| SiO2 | Al2O3 | SiO2 | 3Al2O3·2SiO2 | Al2O3 | SiO2 | low | ||
| MgO | Al2O3 | MgO | MgO·Al2O3 | MgO | MgO·Al2O3 | Al2O3 | moderate | |

XRD results for CaO+Al2O3, FeO+SiO2, FeO+Al2O3, FeO+MgO, SiO2+Al2O3 and MgO+Al2O3 mixture heated at 1373 K under Ar for 1 hour.
The XRD result for CaO+FeO+SiO2 mixture is shown in Fig. 6. The phases observed were FeO, SiO2 and 2CaO · SiO2. It should be mentioned that CaO was likely to be dissolved into FeO phase. The predicted phases at 1373 K for CaO–FeO–SiO2 mixed at same weight ratio is CaO · SiO2 and CaO · FeO · SiO2 from CaO–FeO–SiO2 phase diagram.3) It is clear that the phases present in the sample held at 1373 K for 1 hour is completely different from the predicted phases from phase diagram. Also, 2CaO · Fe2O3 and FeO · SiO2 phases that formed in the previous experiment for CaO+FeO and FeO+SiO2 mixtures were not observed in the ternary oxide mixture. It can be said that the reactivity of CaO and SiO2 to form 2CaO · SiO2 is much higher than reactivity of CaO+FeO and FeO+SiO2 and CaO completely reacted with SiO2 in the present experiment.

XRD results for CaO+FeO+SiO2 mixture heated at 1373 K under Ar for 1 hour.
The XRD results for CaO+FeO+SiO2+Al2O3 and CaO+FeO+SiO2+MgO mixtures are shown in Fig. 7. Semi-quantitative abundance ratio of phases observed after heating at 1373 K for 1 hour for CaO+FeO+SiO2, CaO+FeO+SiO2+Al2O3 and CaO+FeO+SiO2+MgO mixtures are shown in Fig. 8. The observed phases for CaO+FeO+SiO2+Al2O3 mixture were FeO, SiO2, 2CaO · SiO2, Al2O3 and CaO · Al2O3. Since it was found from the experiment using two oxides that reactivity of CaO is high, no CaO was found after heating and all CaO reacted with SiO2 or Al2O3 to form 2CaO · SiO2 or CaO · Al2O3 or dissolved into FeO phase. Also, FeO didn’t react with other oxides. The observed phases for CaO+FeO+SiO2+MgO mixture were SiO2, 2CaO · SiO2, (Fe,Mg)O, 2FeO · Fe2O3 and CaO. Unlike other samples, some CaO remained unreacted.

XRD results for CaO+FeO+SiO2+Al2O3 and CaO+FeO+SiO2+MgO mixture heated at 1373 K under Ar for 1 hour.

Semi-quantitative abundance ratio of phases observed after heating at 1373 K for 1 hour for CaO+FeO+SiO2, CaO+FeO+SiO2+Al2O3 and CaO+FeO+SiO2+MgO mixtures.
Recalling that by heating mixture of CaO+FeO, sample was nearly completely melted, it is highly possible that liquid CaO–FeO phase formed for CaO, FeO containing samples. To clarify the formation mechanism 2CaO · SiO2, additional experiment was conducted. Sample prepared by mixing 0.4 g of CaO, 0.4 g of FeO and 0.2 g of SiO2 was heated at 1373 K for 1 hour under Ar flow. Size of SiO2 powder was increased to 100 μm. The SEM image of the sample after heating is shown in Fig. 9. As seen from Fig. 9, it was confirmed that 2CaO · SiO2 formed at the interface of CaO–FeO liquid phase and SiO2 solid phase. This CaO–FeO liquid phase can react with SiO2 and highly possible to react with Al2O3 to form oxide compounds. Reaction can be expressed as Eqs. (1), (2), (3).
| (1) |
| (2) |
| (3) |
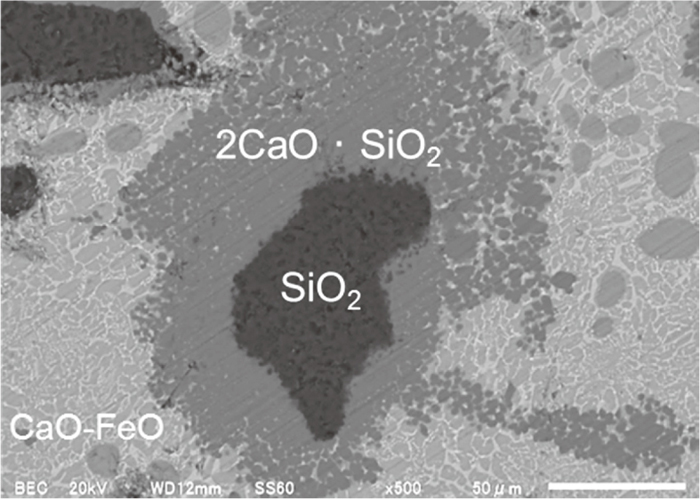
SEM image of sample for CaO+FeO+SiO2 mixture heated at 1373 K under Ar for 1 hour. (Composition: 40 mass%CaO–40 mass%FeO–20 mass%SiO2, Intial SiO2 powder size: 100 μm)
Fundamental reactivity between oxides at 1373 K was investigated in the present work. It was found that the phases present in the sample after heating was different with equilibrium phases for most cases. The reactivity of CaO and FeO was found to be high and Al2O3 was low. Also, formation of CaO–FeO liquid phase will assist the reaction between CaO and other oxides. Addition of MgO will avoid CaO–FeO liquid formation. Information to control slag melting temperature was obtained in the present work.
Fundamental reactivity between oxides at 1373 K under Ar atmosphere was investigated. The phases observed after experiment was different from predicted phase and deviated from equilibrium for most experiment. Reactivity of various oxides was investigated and it was found that reactivity of CaO and FeO was high and Al2O3 was low. Formation of CaO–FeO liquid phase was found when CaO and FeO coexisted and this liquid phase will assist the reaction between CaO and other oxides. Also, it was found that presence of MgO will avoid CaO–FeO liquid formation by rapid reaction to form (Fe,Mg)O solid solution. Important knowledge to control slag melting temperature in blast furnace was obtained in the present work.