2016 Volume 56 Issue 11 Pages 1938-1947
2016 Volume 56 Issue 11 Pages 1938-1947
Effect of Cr2O3 on the reduction mechanism of high-chromium vanadium-titanium pellets was firstly studied in the present paper. It is found that Cr2O3 has an obvious effect on the reduction of high chromium vanadium-titanium pellets. With increasing Cr2O3 content from 0.28% to 8.22%, the reduction extents increased slightly and then decreased, and the reduction mechanism was further elucidated by means of XRD and SEM-EDS. Effect of Cr2O3 on the smelting mechanism of high-chromium vanadium-titanium pellets was also studied in the present paper. With increasing Cr2O3 contents from 0.28% to 8.22%, the softening start temperature and the softening temperature gradually increased, and the softening zone increased as a whole. The melting start temperature increased as a whole. The dripping temperature gradually increased, the melting-dripping zone increased quickly to a relatively high value of above 230°C, and the permeability index increased evidently, indicating the deterioration of melting-dripping index. The dripping difficulty increasing with the increase of Cr2O3 contents is in accordance with the observed Cr-multicarbides and Cr-carbides in the XRD pattern and the microscopic examination of the not-dripped and dripped melted iron and slag.
In Panzhihua-Hongge District, Sichuan Province there are 3.6 billion tons of high chromium vanadium-titanium magnetite reserves with great development value.1,2,3) It is very rewarding to exploit and comprehensively utilize these mineral resources. In this paper, experimental Russia high chromium vanadium-titanium magnetite is similar to those Hongge ores. Thus, it can provide theoretical reference for those unmined ores.
For high chromium vanadium-titanium magnetite, researches have been carried out for several years,3,4,5,6,7) but systematical researches are still scarce. The ores can been used through blast furnace process or direct reduction technology including Midrex, HYL-I, HYL-III, and Rotary hearth. Comparing with the direct reduction technology, the BF process with an efficient countercurrent exchanger of heat, mass, and oxygen is a significant measure and pathway to smelt iron ore such as high chromium vanadium-titanium magnetite on a large scale. Some previous researches have been done on the preparation, property and application in the ironmaking process of high chromium vanadium-titanium pellet and sinter. Zhou et al.3) investigated the influence of MgO in form of magnesite on properties and mineralogy of high chromium vanadium-titanium magnetite sinters with 0.102–0.105 mass -% Cr2O3 contents. Tang et al.4) studied the reduction mechanism of high-chromium vanadium–titanium magnetite pellets by H2–CO–CO2 gas mixtures. Cheng et al.5) investigated the non-isothermal reduction kinetics and mechanism of high-chromium vanadium–titanium magnetite pellets in the lumpy zone of BF, and Liu et al.6) studied the reduction process of pellets containing high-chromium vanadium-titanium magnetite in the cohesive zone. Cheng et al.7) researched the smelting mechanism of TiO2 effects on the crushing strength and smelting mechanism of high-chromium vanadium-titanium magnetite pellets. However, all these Cr2O3 contents of experimental oxidized pellet samples in these studies were lower than 0.6 mass -%, and little attention has been devoted to study the smelting mechanism of pellet samples with relatively higher Cr2O3 contents to reveal the Cr2O3 effects during reduction and smelting.
In this paper, the purpose is further perfecting the high chromium vanadium-titanium smelting mechanism on the basis of having accomplished the study on the effect of TiO2 on the smelting mechanism of high chromium vanadium-titanium pellets,7) and in order to accomplish this purpose, researches were carried out for the effect of Cr2O3 on the reduction mechanism and on the softening-melting-dripping behavior and mechanism of high chromium vanadium-titanium pellets under the simulated blast furnace condition with different characterized means including ICP-AES, XRF, XRD and SEM - EDS described with individual parameters in the subsequent experimental part.
In this study, the oxidized high chromium vanadium-titanium magnetite pellet samples were made with the material proportion of 40% high chromium vanadium-titanium magnetite, 60% ordinary ores and outside with different mass-% Cr2O3 additives of 0, 3%, 6% and 9% and with the oxidation process from 900°C–1275°C and keeping constant at 1275°C for 30 min. All the crushing strength values of pellets of 10–13 mm in diameter were more than 2500N, and the chemical composition of high chromium vanadium-titanium pellets with different mass -% Cr2O3 is shown in Table 1. From the XRD patterns of high chromium vanadium-titanium pellets with 0.28%, 3.11%, 5.85% and 8.22% Cr2O3 contents shown in Fig. 1, it is noticed that Fe-bearing phase is mainly hematite, together with complex oxides with Ti and Cr, which can also be observed in the pellet microstructure shown in Fig. 2. It is obtained that the relative contents of Cr-oxides increased while Fe2O3 correspondingly decreased with increasing the Cr2O3 content in the original high chromium vanadium-titanium pellet samples through the semiquantitative analysis of XRD pattern, which is coincident with the chemical composition in Table 1. Figure 3 presents the elemental compositions of area A, B, C and D marked in Fig. 2. Cr-bearing phases are CrVO3 and (Fe0.6Cr0.4)2O3, and gangue phase was found both in the XRD phases and microstructure analysis. The vitreous body substances composed of silicate minerals and Mg–Al spinel was also detected as area C in the microphotographs.
| Blended Cr2O3 | TFe | FeO | V | TiO2 | Cr2O3 |
|---|---|---|---|---|---|
| 0% | 62.25 | 0.66 | 0.18 | 2.47 | 0.28 |
| 3% | 60.55 | 0.60 | 0.20 | 2.58 | 3.11 |
| 6% | 57.40 | 0.26 | 0.21 | 2.35 | 5.85 |
| 9% | 57.53 | 0.13 | 0.20 | 2.37 | 8.22 |

XRD patterns of high chromium vanadium-titanium pellets with different mass -% Cr2O3 (a) 0.28% (b) 3.11% (c) 5.85% (d) 8.22%.

SEM microphotographs of high chromium vanadium-titanium magnetite pellets with 5.85% mass -% Cr2O3.

EDS analysis of high chromium vanadium-titanium magnetite pellets with 5.85% mass -% Cr2O3.
The reduction kinetic experiments for high chromium vanadium-titanium pellets with different mass -% Cr2O3 were carried out in the apparatus made by Northeastern University, and the reduction reactor was 75 mm in diameter made from stainless steel. Firstly, the high chromium vanadium-titanium pellets with 0.28, 3.11, 5.85 and 8.22 mass -% Cr2O3 were reduced for two hours at 900°C under the atmosphere of 4.5 L/min CO and 10.5 L/min N2. The atmosphere is the same as subsequent softening-melting-dripping experiments, and both are simulating the blast furnace conditions. When reduction ended, the reduced pellets were cooled with 5 L/min argon gas to room temperature and were used to analyze the kinetic data, phase composition and microscopic examination in order to obtain the reduction behavior and mechanism of high chromium vanadium-titanium pellets.
The softening-melting-dripping experiments for high chromium vanadium-titanium pellets with different mass -% Cr2O3 were carried out in the apparatus described previously,6) with the reduction tube of 75 mm in diameter. And the charging method was under simulated conditions of the blast furnace charging, namely, the coke of 20 mm-height was put in the bottom of crucible and 500 g high-Cr V–Ti pellets were put on the nether coke, together with the coke of 40 mm-height on the pellets. After charging, the pressure lever and the charged crucible were put in the reduction tube, and make sure the thermocouple was wired well. In order to guarantee the accuracy of differential pressure, it is of great need to seal hermetically the reduction tube bottom well, preventing the gas leaking. Then the displacement transducer was adjusted to smoothly being pressed on the pressure lever by the external pressure. The pressure can be regulated during experiments by hands according to the loading extent of burden in the descending process. With the preparation work finished, the definite temperature profile and reduction atmosphere in different temperature ranges7,8) shown in Table 2 were adopted till the end of dripping. After dripping, the argon gas was inlet quickly till room temperature to prevent the reduced products from oxidizing. The softening-melting-dripping behavior and mechanism were gained with the exact index for the smelting process and the product analysis of mineral phases and microscopic examination.
| Temperature range | 0–400°C | 400–900°C | 900–1020°C | 1020°C – dripping temperature |
|---|---|---|---|---|
| Heating-up velocity | l0°C/min | l0°C/min | 3°C/min | 5°C/min |
| Heating-up time | 40 min | 50 min | 40 min | >60 min |
| Gas composition | N2 100% | N2 60% 9 L/min | N2 70% 10.5 L/min | |
| 3 L/min | CO 26% 3.9 L/min | CO 30% 4.5 L/min | ||
| CO214% 2.1 L/min | ||||
The chemical compositions of both original pellet samples and different reduced products were analyzed by inductively coupled plasma - Atomic emission spectroscopy (ICP-AES, Optima 8300DV; PerkinElmer, USA) and X-ray fluorescent (XRF, ZSXPrimus II; Rigaku, Japan). The mineral phases of different specimens were studied through X-ray diffractometer (XRD, X’ Pert Pro; PANalytical, Almelo, the Netherlands) with Cu Kα radiation (λ = 1.5406 Å). The microstructure, element compositions and distributions of pellets and reduced products were examined by Scanning electron microscope - Energy Disperse Spectroscopy (SEM-EDS, Ultra Plus; Carl Zeiss GmbH, Jena, Germany).
Figure 4 exhibits typical results for the reduction of high chromium vanadium-titanium pellets with 0.28, 3.11, 5.85 and 8.22 mass -% Cr2O3 at 900°C. It is found that the Cr2O3 contents have a clear effect on the reduction of high chromium vanadium-titanium pellets. The reduction extents increased slightly with increasing Cr2O3 content from 0.28% to 3.11% with the same reduction time, and then decreased with increasing up to 8.22%. In Fig. 5 the XRD pattern of high chromium vanadium-titanium pellets after reduction at 900°C for two hours with 0.25%, 3.11%, 5.85% and 8.22% Cr2O3 is shown. On one hand, it is found that with increasing Cr2O3 content from 0.28% to 3.11%, the extent of iron oxide reduction increased as the peak of FeO at 2-Theta of 42.0° relatively went down while the peak of Fe at 44.7° relatively went up. In U. F. Chinje, et al.’s study10) it has been stated that as Cr3+ has relatively stronger chemical adsorption to CO, certain catalysis for the reduction reaction can be intensified in certain ranges of Cr2O3 in original high chromium vanadium-titanium pellets, which further validates the slight increase of reduction extent with increasing Cr2O3 from 0.28% to 3.11%. With increasing Cr2O3 content from 3.11% to 8.22%, the extent of iron oxide reduction decreased not only as the integrated peaks of FeO at 2-Theta of 42.0° and of Fe3O4 at 2-Theta of 43.1° relatively went up while the peak of Fe at 44.7° relatively went down but also as the peak of unreduced Fe3O4 which was reduced from Fe2O3 at 2-Theta of 30.1°, 35.5°, 43.1° and 57.1° relatively went up. On the other hand, with the increase of Cr2O3 contents, the reduction of Cr-oxides should also be paid great attention. By analyzing the phase composition transformation of high chromium vanadium-titanium pellets before and after reduction with Figs. 1 and 5, it is found that most Cr-bearing oxides as Cr–Fe spinel named FeCr2O4 (FeO·Cr2O3) transformed from the original (Fe0.6Cr0.4)2O3 remained unreduced at the end of the reduction experiment. According to the Eqs. (1) and (2), FeCr2O4 is difficult to be reduced.
| (1) |
| (2) |

Kinetic data for reduction of high chromium vanadium-titanium pellets with different mass -% Cr2O3.

XRD pattern of reduced high chromium vanadium-titanium pellets at 900°C for two hours with different mass -% Cr2O3 (a) 0.28% (b) 3.11% (c) 5.85% (d) 8.22%.
Only tiny amounts of the reduced product as metal Cr was found in the XRD patterns of reduced pellets in the cold lumpy zone, which is probably due to the fact that the high reduction potential condition as the ratio of CO/CO2 could be regarded as infinitely great for some certain time. In addition, it has been reported that in KHEDR’s studies (Fe,Cr)2O3 was found to be a significant factor that affect the rate of reduction,9) which further validates the affecting factor of Cr–Fe spinel.
Figures 6 and 7 exhibit the SEM microphotographs of reduced high chromium vanadium-titanium pellets with 5.85% and 8.22% Cr2O3 at 900°C for two hours at 100×, 500× and 2000×. It is observed that the generated metallic iron has the tendency of accumulation, and the tendency is more obvious for reduced pellets with less Cr2O3 contents. The EDS analysis of area A, B, C and D in the reduced high chromium vanadium-titanium pellets with 8.22% Cr2O3 at 900°C for two hours is presented in Fig. 8. Few amount of metal Cr was reduced from Cr-bearing oxides and transformed into the metallic iron (area A) observed in the EDS results in Fig. 8(a). Previous studies have shown that metal Cr is apt to stay with metallic iron.1,7) Once reduced metal Cr appeared, it is easily found in the metallic iron. Most Cr components, examined in the light grey areas like area B and C with the EDS analysis shown in Figs. 8(b) and 8(c), was still in the incompletely reduced Cr–Fe oxides. The gangue phases (area D) still existed in reduced high chromium vanadium-titanium pellets. By surface scanning presented in Fig. 9, the distribution of Cr in the reduced pellet microstructure can be seen evidently, conducive to further studying the reduction and transformation behavior.
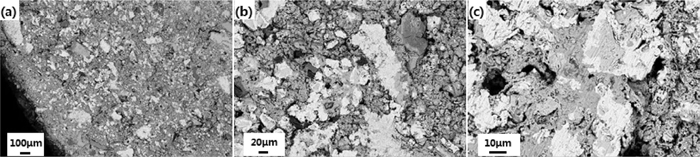
SEM microphotographs of reduced high chromium vanadium-titanium pellets with 5.85% Cr2O3 at 900°C for two hours.

SEM microphotographs of reduced high chromium vanadium-titanium pellets with 8.22% Cr2O3 at 900°C for two hours.

EDS analysis of reduced high chromium vanadium-titanium pellets with 8.22% Cr2O3 at 900°C for two hours.

Element distributions of reduced high chromium vanadium-titanium pellets with 8.22% Cr2O3 at 900°C for two hours.
Figure 10 show the typical results of T4, T40 and softening zone of furnace burdens with different Cr2O3 contents. T4, defined as softening start temperature, is the temperature that the contraction percentage of the furnace burden is 4%, and T40 defined as softening temperature, is the temperature that the contraction percentage of the furnace burden is 40%. The softening zone is defined as the temperature different value of softening temperature and softening start temperature, namely T40–T4. It is found that the softening start temperature gradually increased from 1088°C to 1160°C with increasing Cr2O3 contents and the softening temperature increased from 1201°C to 1295°C. The softening zone increased from 113°C to above 135°C with the increase of Cr2O3 from 0.28% to 8.22% as a whole. As is well known, the phenomenon that softening start temperature increasing is good for the stability of the furnace condition and the proceeding of gas-solid reduction reaction. It is found that the softening zone kept the increasing tendency, and correspondingly the softening index kept the deterioration trend as the softening zone increasing negatively favors the furnace condition and the proceeding of gas-solid reduction reaction. With the increase of Cr2O3 contents, the iron oxides combined with chromium oxides increased, and the Cr–Fe oxides is much more difficult to be reduced than separate iron oxides according to the thermodynamic analysis. In the above text, the reduction kinetic data also elucidated this reduction condition.

T4, T40 and softening zone for different furnace burdens.
The typical results of Ts, Td, and melting-dripping zone (Td–Ts) of furnace burdens with different Cr2O3 contents are presented in Fig. 11. Ts, defined as melting start temperature, is the temperature accompanying differential pressure with a massive jump, and Td, defined as dripping temperature, is the temperature that the pig iron drips from the graphite crucible. It is gained that the melting start temperature gradually increased from 1223°C with the Cr2O3 content of 0.28% to 1328°C with the content of 5.85%, and then decreased to 1313°C with the content of 8.22%. The increase of melting start temperature is propitious to the BF strengthening smelting. The dripping temperature increased from 1338°C to 1570°C with increasing the Cr2O3 content from 0.28% to 8.22%. However, the melting-dripping zone increased quickly to a relatively high value of above 230°C, indicating the deterioration of melting-dripping index. Thus, the position distribution of melting-dripping zone was obtained with the increase of Cr2O3 contents, with the results shown in Fig. 12, in which the phenomenon that the position wholly shifted down improved the blast furnace smelting index but that the zone widened reversely improved the index.

Ts, Td and melting zone for different furnace burdens.

The position distribution of melting-dripping zone for different furnace burdens.
There are many factors influencing the melting-dripping characteristics, and one important key factor is the property of Cr-bearing molten iron and Cr-bearing slag besides the effect of Ti(C,N) elucidated in the previous study. From the ternary phase diagram of Fe–Cr–C, it can be seen that the liquidus temperature is relatively higher, so the melting point and fluidity of Cr-bearing molten iron is worse than ordinary molten iron which has also been reported that in Liu’s study.11) Furthermore, the meting point and fluidity increases with increasing Cr contents in molten iron.12) As the radius of Cr atom is bigger than Fe atom, the free space of iron melts decreases with the increase of Cr contents in molten iron, and this contributes to the decrease of fluidity and the increase of viscosity of molten iron. The effect of Cr on the fluidity and viscosity in the Fe–Cr–C system has also relations with the precipitated temperature of Cr-carbides and the precipitated forms.11) Cr-Multicarbides of (Cr,Fe)23C6, (Cr,Fe)3C2 and (Cr,Fe)7C3 can be found when the melted iron existed in the Fe–Cr–C system, and it has been reported that Cr-carbides are easier to generate than metal Cr in the reduction process of Cr2O3.13) The Cr-multicarbides (Cr15.58Fe7.42C6) and Cr-carbides (CrC) were found through the XRD analysis shown in Fig. 13 for the dripped products of blast furnace burdens with higher Cr2O3 contents of above 3.11%. Furthermore, it was found that the amount of Cr-multicarbides and Cr-carbides increased with increasing Cr2O3 contents. In addition, unreduced Cr2O3 in the BF slag could increase the viscosity of the Ti-bearing blast furnace slag as it has been reported that Cr2O3 in the BF slag could easily react with MgO and Al2O3 to generate spinel phase of MgCr2O4 and MgCrAlO4 with high melting points.14)

XRD pattern of dripped products for pellet furnace burdens with 3.11% and 5.85% Cr2O3.
In order to further study the smelting mechanism of Cr-bearing furnace burdens, other factors were also gained. Table 3 presents the indexes including maximum differential pressure and corresponding temporary temperature, dripping differential pressure and permeability index for the melting-dripping process of different furnace burdens. It is observed that the maximum differential pressure firstly decreased to 14946 Pa with 3.11% Cr2O3, and then increased to 23206 Pa with increasing the Cr2O3 content to 8.22%. The temperature that the maximum differential pressure reached increased obviously from 1291°C to 1474°C with the increase of Cr2O3 contents. The Dripping differential pressure increased as a whole. The permeability index namely S value, calculated according to the parameters of the melting-dripping process, increased evidently from 1232343 Pa·°C to 2588504 Pa·°C with increasing Cr2O3 contents, and the increasing rate of the permeability index was greatly much more quicker compared with that with increasing TiO2 contents.7) The permeability index was in a much higher value when Cr2O3 content exceeded 5.85% than those for both ordinary pellets without TiO2 and Cr2O3 and ordinary vanadium-titanium magnetite pellets.7,15) The permeability index reflects the viscosity of Cr-bearing melted iron which influences the fluidity of the melted iron. As the dripping difficulty of ordinary Ti-bearing melted iron is serious, the dripping difficulty could be much greater with the Cr addition, which has been reflected in the melting-dripping characteristics and this permeability index. The effect of Cr2O3 on the smelting mechanism for high-chromium vanadium-titanium pellet burdens could be paid greater attention than the effect of TiO2 for the pellet burdens.
| Cr2O3 content | ΔPmax/Pa | TΔP/°C | Dripping differential pressure/Pa | S value/Pa·°C |
|---|---|---|---|---|
| 0.28% | 17722 | 1291 | 1448 | 1232343 |
| 3.11% | 14946 | 1366 | 2775 | 1599833 |
| 5.85% | 17130 | 1469 | 2949 | 2033204 |
| 8.22% | 23206 | 1474 | 2853 | 2588504 |
In Figs. 14 and 15 the microphotographs of dripped melted iron with 3.11% and 5.85% Cr2O3 are presented at 500×, 3000×, 5000× and 10000× respectively. The area C is the pore with melted iron in. It is found that the size of the melted iron decreased with the increase of Cr2O3 contents. As is well-known, the bigger the size of the melted iron, the smaller the dripping difficulty. The above-mentioned dripping difficulty increasing with the increase of Cr2O3 contents is in accordance with the microscopic examination. By analyzing the element distributions of area A, B and C, the EDS analysis results were obtained, exhibited in Figs. 16 and 17. It is observed that the contents of V and Cr were in a relatively higher level considering the V content in the original furnace burden, while the content of Ti is in a relatively lower level. From the chemical composition analysis of dripped products for high-Cr vanadium-titanium pellet furnace burdens with 3.11% and 5.85% Cr2O3 shown in Table 4, it can also be found that V and Cr ratios in the melted iron is clearly more than those in the slag while the Ti ratio in the melted iron is clearly less than those in the slag. In the reduction process of vanadium and chromium oxides to corresponding hot metal V and Cr, with the accumulation of melted iron, the amount of Cr and V in melted iron increased. On the other hand, as titanium oxides is impossible to be reduced into hot metal Ti in the present blast furnace conditions, with the accumulation of melted iron, the obvious Ti amount increase cannot be found. The transformation behavior of valuable components including Cr, V and Ti has also been proposed that Cr and V are more apt to move to melted iron while Ti is more apt to move to slag with the formation of melted iron and slag in the previous study.1,7) It is further validated with present EDS analyses and chemical compositions with much more Cr contents in the original smelting furnace burdens. In order to investigate the total element distribution, the surface scanning was analyzed. Figure 18 presents the element distributions of dripped melted iron for pellet furnace burden with 5.85% Cr2O3. Compared with the surface scanning of dripped melted iron for pellet furnace burden with 0.24% Cr2O3,7) it can be seen clearly that the Cr distribution increased clearly to a larger extent. It is also observed that Cr and V are relatively more evenly distributed in the melted iron while Ti is relatively more dispersedly distributed. Most generated Ti-bearing substances like TiC and TiN accumulated on the coke surface due to the favorable wettability of Ti(C,N) and coke.1) The formation of Ti(C,N) is almost unavoidable, and it was also found much in the not-dripped products for pellet furnace burden with 5.85% Cr2O3 presented in Fig. 19. Owing to the appearance of Ti(C,N), the slag and melted iron were easily combined together and difficult to separate. By separating the not-dripped slag-iron for pellet furnace burden with 3.11% Cr2O3 at 1400°C for 1 h, only tiny slag were separated and there was still melted iron in the tiny amount of the separated slag which can be found in the SEM microphotograph of separated iron shown in Fig. 20.

SEM microphotographs of dripped melted iron for pellet furnace burden with 3.11% Cr2O3 (a) at 500× (b) at 3000× (c) at 5000× (d) at 10000×.

SEM microphotographs of dripped melted iron for pellet furnace burden with 5.85% Cr2O3 (a) at 500× (b) at 3000× (c) at 5000× (d) at 10000×.

EDS analysis of dripped melted iron for pellet furnace burden with 3.11% Cr2O3.

EDS analysis of dripped melted iron for pellet furnace burden with 5.85% Cr2O3.
| Cr2O3 | TFe | V | Cr | Ti |
|---|---|---|---|---|
| 3.11% | 91.63 | 0.22 | 2.37 | 0.028 |
| 5.85% | 90.26 | 0.211 | 3.87 | 0.047 |

Element distributions of dripped melted iron for pellet furnace burden with 5.85% Cr2O3.

SEM microphotographs of not-dripped product for pellet furnace burden with 3.11% Cr2O3 (a) at 500× (a) at 2000×.

SEM microphotographs of separated slag from not-dripped slag-iron for pellet furnace burden with 3.11% Cr2O3 (a) at 2000× (a) at 10000×.
Effect of Cr2O3 on the reduction mechanism and smelting mechanism of high-chromium vanadium-titanium pellets was studied in the present paper. Following conclusions can be drawn:
It is found that Cr2O3 has an obvious effect on the reduction of high chromium vanadium-titanium pellets. With increasing Cr2O3 content from 0.28% to 8.22%, the reduction extents increased slightly and then decreased, and through the XRD analysis, SEM examination and EDS analysis the reduction mechanism was further elucidated.
With increasing Cr2O3 contents from 0.28% to 8.22%, the softening start temperature and the softening temperature gradually increased, and the softening zone increased as a whole. The melting start temperature increased as a whole. The dripping temperature gradually increased, the melting-dripping zone increased quickly to a relatively high value of above 230°C, and the permeability index increased evidently, indicating the deterioration of melting-dripping index. The dripping difficulty increasing with the increase of Cr2O3 contents is in accordance with the observed Cr-multicarbides and Cr-carbides in the XRD pattern and the microscopic examination of the not-dripped and dripped melted iron and slag.
The authors are especially thankful to the Major Program of National Natural Science Foundation of China (Grant No. 51090384), 863 Program (Grant No. 2012AA062304) and Fundamental Research Funds for the Central Universities (Grant No. N150203003 and No. N150202001).
ΔGθ: standard Gibbs free energy of formation [J·mol−1]
T4: softening start temperature [°C]
T40: softening temperature [°C]
Ts: melting start temperature [°C]
Td: defined as dripping temperature [°C]
ΔPmax: maximum differential pressure [Pa]
TΔP: corresponding temporary temperature when the differential pressure is maximum [°C]
S: permeability index [Pa·°C]