2016 Volume 56 Issue 12 Pages 2118-2125
2016 Volume 56 Issue 12 Pages 2118-2125
CF is regarded as the best bonding phase with superior strength and reducibility in sintering. The comparison of hematite and CF reduction process was made in this study. Isothermal reduction experiments of powdered hematite and CF in a continuous stream of 30% CO and 70% N2 at 1123 K, 1173 K and 1223 K were conducted through thermo-gravimetric analysis (TGA). Reduction rate analysis revealed that the reduction of hematite comprises three independent steps (H→M→W→I), whereas that of CF mainly comprises two steps (H→M→I). The reduction of powdered hematite and CF can be described by shrinking layer model, not shrinking core model completely. Results of the ln-ln and Sharp analysis methods indicated that the reduction of hematite included two kinetics stages, namely, plane-like mechanism and then spherulitic-type mechanism. The reduction of CF only included the plane-like mechanism stage. The model free method revealed that the activation energy of hematite and CF reduction were 5.81 kJ·mol−1 and 46.89 kJ·mol−1, respectively.
Fluxed sinters are commonly used raw materials for the iron ores of a blast furnace. Moreover, silico-ferrite of calcium and aluminum (SFCA) is treated as the best bonding phase in the fluxed sinter. A sinter with superior reducibility decreases the fuel rate of ironmaking in a blast furnace, and provides iron-rich raw material with a suitable porosity to facilitate the smooth operation of blast furnace. Essentially, the reduction of iron ore sinter with high-basicity is determined by the reducibility of iron oxide and SFCA. The comprehensive examinations on the reduction of SFCA and the qualitative comparisons of iron oxide with SFCA have great significance to investigate the iron ore sinter but were gained few attention from previous study. The present study discusses the reduction behavior of pure and binary calcium ferrite (CF) in comparison with that of hematite.
The reduction of CF is a typical gas-solid reaction chemically regarded as a gaseous reduction reaction of iron oxide.1,2,3,4,5) Ganguly6) analyzed the reduction behavior of pure calcium ferrites (calcium ferrite, calcium diferrite and dicalcium ferrite) in H2, CO and CO–CO2 gas mixtures. Maeda et al.7) studied the final stage of reduction of quaternary calcium ferrite with CO–CO2 gas mixtures. Weiss et al.8) investigated the reduction process of hematite to magnetite and found that the system gradually forms a micro-porous layer of magnetite while the porosity initially increases and then decreases. Turkdogan et al.9,10,11,12) explored the gaseous reduction of iron oxides in H2 or CO–CO2 mixtures and discussed the rate-controlling step of reduction process. However, the comparison of reduction behavior of hematite and CF at same test conditions remains poorly understood. Thus, the present study investigated the isothermal kinetics of hematite and CF in CO–N2 gas mixtures through thermo-gravimetric analysis (TGA).
The first purpose of this study is to compare the reducibility of solid phase in the CaO–Fe2O3 system, as CF and Fe2O3 are main reduced phases in the CaO–Fe2O3 system, otherwise, the powdered samples were rarely studied aiming at mechanism descriptions. The influence of particle size on the reduction of iron ore was studied, Fine particles in the blast furnace have different reduction behavior with lump ores. It is known that powdered samples with larger porosity make reduction gas diffuse easily than pellet samples. The reduction of iron oxide pellet can be explained by the shrinking core model, in which CO (or H2) diffuses from the iron layer to the hematite layer to trigger the reduction of wustite to iron, magnetite to wustite, and hematite to magnetite, but whether or not the model is suitable for the powdered samples in this study requires further assessment, that is another purpose of this paper. In addition, as a series work, the reduction kinetics of CaO–Fe2O3–x systems (x: SiO2, Al2O3 and MgO) has been finished and are prepared to be summarized into the system of SFCA.
CF samples were prepared from CaCO3 (≥99.99%) and Fe2O3 (α-Fe2O3, ≥99.99%) at a 1:1 molar ratio. The powdered raw materials were uniformly mixed and then pressed to cylindrical shape samples. The samples were roasted in a Si–Mo furnace to 1173 K (900°C) and held for 1 h to decompose CaCO3 to CaO and then increased to 1473 K (1200°C) for 10 h to allow the complete formation of CF. The entire process was carried out in air. The reacted samples were ground into powder for the next investigation. The powder size distribution of hematite and CF were analyzed by laser particle test shown in Fig. 1.

Powder size of hematite and CF by laser particle test.
X-ray diffraction (XRD) using D/max2500/PC (Cu Kα) was performed to ensure the phase composition of the roasted samples. The XRD result shown in Fig. 2 indicates that CF is the main phase of the samples, and quantitative analysis presents that the samples contain CF with approximately 93 mass%.
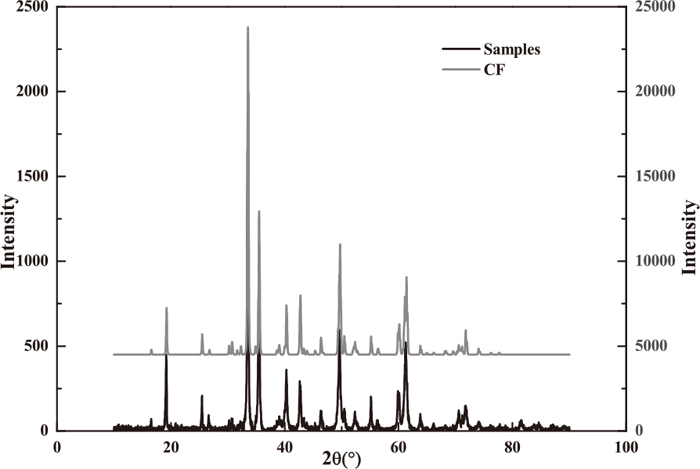
XRD patterns of samples and CF in standard PDF card.
TG measurement of the reduction kinetics of powdered hematite and CF was conducted using a Setaram analyzer (Model Setsys Evo TG-DTA 1750) shown in Fig. 3. The samples (20 mg) were heated in alumina crucible to 1123 K (850°C), 1173 K (900°C) and 1223 K (950°C) in N2 atmosphere at 15 K·min−1. When the temperature reached a fixed value, CO-30% and N2-70% gas mixtures at 20 and 30 ml·min−1 were blown into the samples to evaluate the effect of external diffusion on the reduction of hematite and CF and allow the complete isothermal reduction of all samples. In order to exclude the influence of system error from thermal analyzer and buoyance force from gas mixtures, blank test at same eliminated conditions with no samples in alumina crucible was conducted. The weight loss data were obtained during the isothermal reduction stage from which the TG data of blank test were deducted.
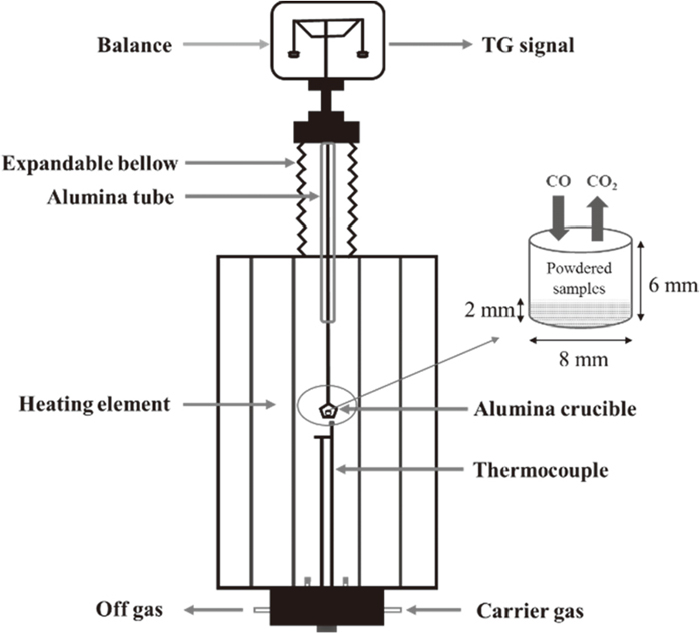
Schematical setup of TG analyzer.
Weight loss from hematite and CF reduction was chemically caused by the removed oxygen from iron oxide. Reduction degree, which is defined as the reaction proceed to a certain extent in terms of time, can be expressed as
| (1) |
Two flow rates of gas mixtures was settle to exclude the effect of external diffusion on the reduction of hematite and CF as Fig. 4-a shows. The difference values of TG data reduced at 20 and 30 ml/min are not more than 2% and 1% for hematite and CF respectively. The results indicate the experimental schedule of samples reduced by gas mixtures at 20 ml·min−1 can nearly exclude the effect on the reduction process of hematite and CF. The weight loss data were easily gained by TG measurement. The changes in reduction degree with time for hematite and CF are described in Fig. 4-b. The oxygen removed from hematite and CF at 1123 K (850°C), 1173 K (900°C) and 1223 K (950°C) reaches almost 30 and 20 mass%, which are almost close to the theoretical values. For the whole hematite reduction process, the weight loss and reduction degree are not obviously different among the three temperatures, but different for that of CF especially during the middle and final stages. The samples show different weight loss behaviors at the same temperature for hematite and CF reduction. Clearly, CF reduction needs more time to complete than hematite reduction. That is, the completion of hematite reduction needs 35 min at 1123 K (850°C), 1173 K (900°C) and 1223 K (950°C), whereas that of CF reduction needs almost 140 min. Raising the temperature cannot obviously promote the hematite reduction process under the experimental conditions.

Weight loss for reduction of hematite and CF at 1223 K (950°C) (a: hematite; b: CF).
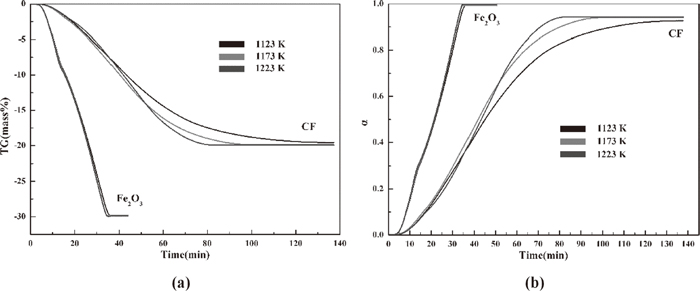
Weight loss and reduction degree of hematite and CF at 850 1123 K, 1173 K and 1223 K (a: weight loss, b: reduction degree).
The calculation of the reduction rate dα/dt is shown in Fig. 5, in which the reduction rate of hematite is much faster than that of CF at the same reduction degree. This result indicates that the reduction of hematite is easier to achieve than that of CF and explains why the reduction degree of hematite could reach almost 1 but not that of CF when the time is extended.
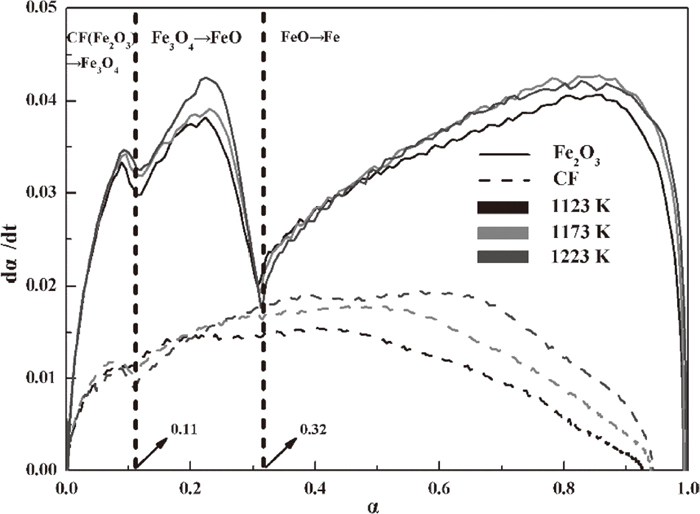
Reduction rate versus reduction degree of hematite and CF.
The reduction steps of hematite and CF can be expressed as
| (2) |
| (3a) |
| (3b) |
| (3c) |
The kinetics basic equation13) that expresses the reduction rate as a function of temperature is shown as follows:
| (4) |
| (5) |
| (6) |
| (7) |
Two analysis methods may be applied to target G(α): ln-ln analysis4) and Sharp analysis.16)
According to the Avrami-Erofeev5,17,18) equation, the reduction degree α can be expressed as the relationship of time t and rate constant k:
| (8) |
| (9) |
| (10) |
| Functions | G(α) | n |
|---|---|---|
| D1(α) | α2=kt | 0.62 |
| D2(α) | (1−α)ln(1−α)+α=kt | 0.57 |
| D3(α) | 0.54 | |
| D4(α) | (1−2/3α)−(1−α)2/3=kt | 0.57 |
| F1(α) | −ln(1−α)=kt | 1 |
| R2(α) | 1−(1−α)1/2=kt | 1.11 |
| R3(α) | 1−(1−α)1/3=kt | 1.07 |
| A2(α) | [−ln(1−α)]1/2=kt | 2 |
| A3(α) | [−ln(1−α)]1/3=kt | 3 |
Sharp analysis defines a non-dimensional parameter y(α) as
| (11) |
The relationship between ln[−ln(1−α)] and lnt for hematite and CF was determined through ln-ln analysis and is presented in Fig. 6. The Avrami exponents n can be calculated as the slope of the plots of ln[−ln(1−α)] against lnt shown in Fig. 7. The n values in Table 2 for hematite reduction can be divided into two stages. hematite reduction can be described by function A2 when the reduction degree α lies between 0.15 and 0.62 and by function A3 when α lies between 0.62 and 0.9. Meanwhile, CF reduction can be only expressed by function A2 as the n values for the entire process are approximately 2.
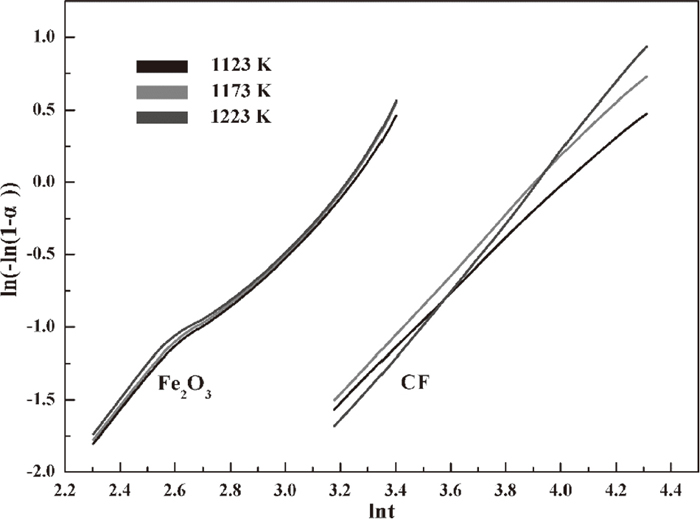
ln[−ln(1−α)] versus lnt at based on ln-ln analysis for hematite and CF.

Avrami exponents n versus reduction degree α for hematite and CF.
| Stages | α | n | Functions |
|---|---|---|---|
| 1 | 0.15–0.62 | 1–2.5 | A2(α) |
| 2 | 0.62–0.9 | 2.5–4 | A3(α) |
| T/K | 1123 | 1173 | 1223 |
|---|---|---|---|
| n | 1.31–2.03 | 1.48–2.17 | 1.87–2.63 |
| α | 0.15–0.8 | 0.15–0.87 | 0.15–0.9 |
| Function | A2(α) | ||
Sharp analysis is another method to confirm the ln-ln analysis result. The standard curves of the plots of y(α) against α and experimental data [α, y(α)] are shown in Fig. 8. The y(α) values for hematite reduction lie at the curve based on function A2 when α is less than 0.6 and then gradually tend to lie at the curve based on function A3. The experimental data y(α) values for CF reduction mostly lie at the standard curve corresponding to function A2. The Sharp analysis results markedly agree with the ln-ln analysis results.
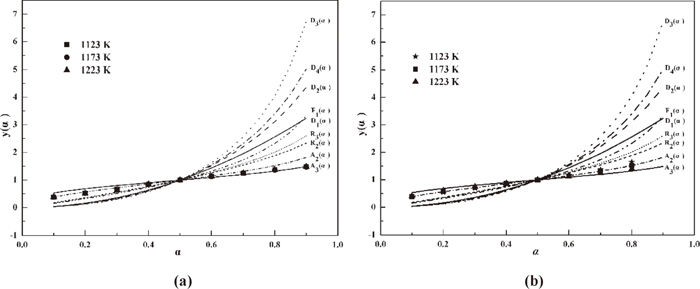
Standard curves and experimental data based on Sharp analysis for hematite and CF (a: hematite, b: CF).
That is, the hematite reduction is controlled by a plane-like mechanism when the reduction degree values are lower than 0.62 and then determined by a spherulitic-type mechanism at the later stage. At the initial stage of hematite reduction, the hematite to magnetite reduction finishes as the reduction degree reaches 0.11 at a fast rate, and the CO content cannot increase up to the target value. Thus, the kinetics of this stage was not discussed in this study. Only the magnetite reduction was included in the discussion. The powdered samples were assumed to be the un-reacted layer, the magnetite layer appeared much larger than the periphery layers when the reaction occurred. As described in Fig. 9(a), the radius of the magnetite layer R is much higher than the distance of the CO diffusion d, and the decreased radius r from the magnetite to wustite reduction is still much less than R. This result indicates that the reduction process occurs in a 2D plane compared with the much larger unreacted layer (magnetite layer). As CO continuously diffuses, the magnetite layer gradually shrinks with decreasing R [Fig. 9(b)]. The R value is much lower than the d value, and the decreased radius r from the magnetite to wustite reduction is much higher than before. This result reveals that the reduction process undergoes a 3D spherulitic-type mechanism.

Reaction mechanism of hematite and CF reduction.
CF reduction is controlled only by a plane-like mechanism. It is caused by a low reduction rate, which makes the decreased radius r always much lower at all stages of CF reduction. Thus, the reduction mechanism is always of the 2D plane type.
In the shrinking core model shown in Fig. 10, which was initially derived from a single ore particle or ore pellet. The core is divided into several homogeneous and interphase layers, in which hematite, magnetite, wustite, and iron are homogeneous layers, and hematite-magnetite, magnetite-wustite, and wustite- iron are interphase layers. CO initially diffuses across the iron layer to reach the wustite-iron interphase layer, and a part of CO reacts with wustite to generate CO2, which diffuses out of the wustite-iron layer with the opposite direction of CO diffusion. The rest of CO continues to diffuse across the wustite layer to reach the magnetite-wustite interphase layer, and a part of CO still reacts with magnetite to generate CO2, which diffuses out of the magnetite-wustite layer as before. The remaining CO finally attaches to the hematite-magnetite layer and reacts with hematite to generate CO2. The unreacted core gradually shrinks as the reaction continues. The reaction rate based on this model only appears one rate peak for all the reduction stages. Figure 5 shows that the reduction of powdered hematite differs from the situation described by the shrinking core model that the magnetite (M) to wustite (W) reduction only occurs after the hematite (H) to magnetite (M) reduction finishes, and the wustite (W) to iron (I) reduction only occurs after the magnetite (M) to wustite (W) reduction finishes, the three reduction stages proceed independently distinguishing from the reaction style based on the shrinking core model.

Shrinking core model for hematite reduction.
Obtaining the natural logarithm on either side of the equal sign of Eq. (6), we can formulate the following equation:
| (12) |
| (13) |
| (14) |
| (15) |
| (16) |
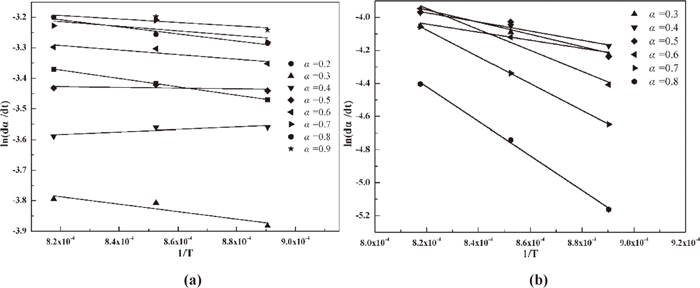
ln(dα/dt) versus 1/T based on model-free mothed for hematite and CF (a: hematite, b: CF).
| α | 0.2 | 0.3 | 0.4 | 0.5 | 0.6 | 0.7 | 0.8 | 0.9 | Avg |
|---|---|---|---|---|---|---|---|---|---|
| E/(kJ·mol−1) | 29.58 | 20.59 | 8.74 | −0.76 | 5.81 | 9.22 | 7.05 | 7.32 | 10.94 |
| α | 0.3 | 0.4 | 0.5 | 0.6 | 0.7 | 0.8 | Avg |
|---|---|---|---|---|---|---|---|
| E/( kJ·mol−1) | 20.32 | 23.11 | 30.85 | 52.85 | 67.51 | 86.68 | 46.89 |
Clearly, the activation energy of hematite reduction is much less than that of CF reduction, which indicates that the former reaction proceeds more easily than the later reaction. This result is in line with the previous conclusion. The activation energy of iron oxide reduction has been calculated by many studies (Table 4). References 19), 20), and 21) researched the solid reduction of hematite by CO or H2 and found higher activation energy than this study, which may be caused by the higher purity and finer particles of hematite in this study. Reference 22) reflected the liquid reduction of hematite by CO.
The Arrhenius equation method can also be used to calculate the activation energy. Considering the complex stages in hematite reduction, we only applied this method for CF reduction. The rate constant k(T) was obtained by finding the plots of G(α) against time t (Fig. 12). The k(T) values at 1123 K (850°C), 1173 K (900°C) and 1223 K (950°C) are listed in Table 5. The fit coefficients R2 are all above 0.99. Higher temperatures mean larger values of k(T).

K(T) calculated with fitting of G(α) versus t.
| T/K | 1123 | 1173 | 1223 |
|---|---|---|---|
| k(T) | 0.0162 | 0.0196 | 0.0238 |
| R2 | 0.9976 | 0.9982 | 0.9987 |
Determined by the Arrhenius equation, the activation energy can be obtained by the slope of fitting of lnk(T) against 1/T in Fig. 13. The activation energy is 43.74 kJ·mol−1 for this method. The pre-exponential factors at 1123 K (850°C), 1173 K (900°C) and 1223 K (950°C) on the basis of the model-free method and the Arrhenius equation method for CF can be determined by Eq. (5) (Table 6).
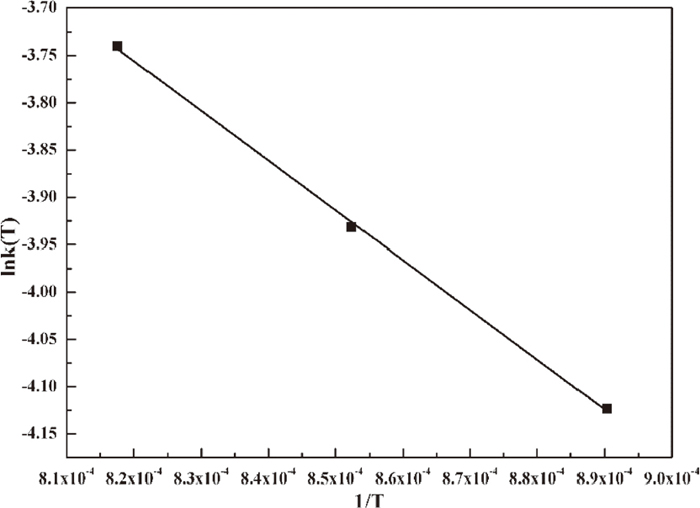
E calculated with fitting of lnk(T) versus 1/T.
| A/(min−1) | ||||
|---|---|---|---|---|
| T/K | 1123 | 1175 | 1223 | Avg |
| Model-free mothed | 2.45 | 2.40 | 2.39 | 2.41 |
| Arrhenius equation mothed | 1.75 | 1.74 | 1.75 | 1.75 |
The reduction of hematite in this study can be described as three independent stages which shows that in the powdered samples all the H transformed to M, all the M to W and then all the W to I. The reduction of hematite at the experimental condition is distinguished from that of pellet iron oxide described by shrinking core model which caused by the powdered samples adopted but not pellet shown in Fig. 14. Large amounts of pore in the powdered samples has the CO diffused into samples smoothly, the reduction H to M proceeding rapidly goes along with the CO diffusion, this reduction stage for all samples completes before the reduction M to W occurs, however, in the shrink core model, the CO diffusion is blocked by the dense pellet, the reduction H to M cannot completes when the reduction M to W occurs. As the pore exists in the powdered samples, the CO diffuses not only in the macroscopic surface but also the particles pore, however, the pellet samples only reduced by CO in the macroscopic surface. It is nearly at the same time that chemical reaction occurs with CO diffusion in the reduction of powdered samples, whereas the chemical reaction lags behind CO diffusion in the pellet reduction. That is why in the shrinking core model that H reduced to M, then to W and finally to I only occur in the surface of unreacted core, not in the inner of particles. The reduction rate appears three peaks in the reduction of powdered samples but only one peak in the pellet reduction. In other words, the reduction of powdered samples of hematite and CF in this study can be described by shrinking layer model not the shrinking core model.
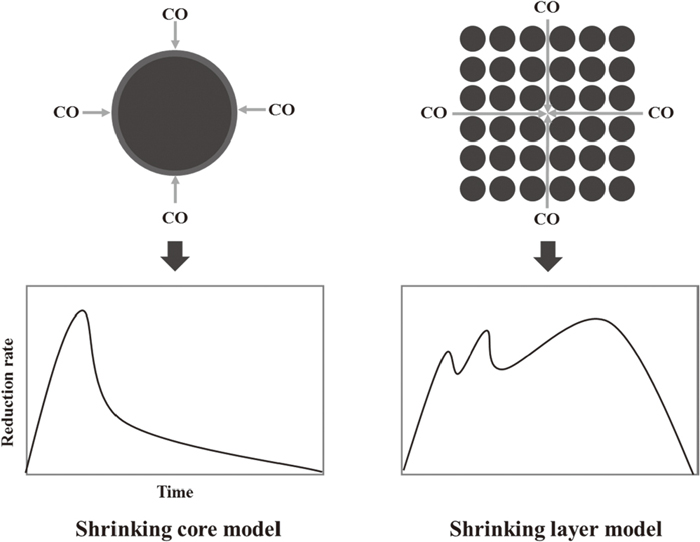
Reduction of powdered described by shrinking layer model.
Temperature mainly contributes to the interphase reaction in the reduction process. As Fig. 5 shows, the effect of temperature on the reduction rate of hematite was not obvious compared with that of CF, especially at the middle and final stages of the reduction process. It supposes that CO inner diffusion is the rate-controlling step at the entire and initial stages of hematite and CF reduction, whereas interface chemical reaction is rate-controlling step at the middle and final stages of CF reduction.
The connection of the rate-controlling step of reduction rate to the activation energy of iron oxide was investigated by Nasr23) (Table 7). As for the hematite reduction process, the E values are all less than 29 kJ·mol−1. It speculated that CO diffusion is the rate-controlling step of the entire process of hematite reduction. The activation energy of CF reduction lies within 20–86 kJ·mol−1 with reduction degree increasing. Hence, the rate-controlling step of CF reduction are performed as inner gas diffusion, inner gas diffusion and interface chemical reaction mixed control then interface chemical reaction in turns with reduction degree increasing. These conclusions agree with the previous discussion. However, the discussion is not the study conclusion and needs more future work in next investigation.
| E/(kJ·mol−1) | Rate-controlling step |
|---|---|
| 8–16 | Inner gas diffusion |
| 29–42 | Inner gas diffusion and interface chemical reaction mixed |
| 60–67 | Interface chemical reaction |
| >90 | Solid diffusion |
The isothermal kinetics for powdered hematite and CF reduction under CO atmosphere by TG measurement was determined in 30% CO and 70% N2 atmosphere. This study has reached the following conclusions:
(1) The results of the ln-ln analysis and Sharp methods indicate that the reduction of hematite includes two kinetics stages, namely, plane-like mechanism and spherulitic-type mechanism. The reduction of CF is determined only by the plane-like mechanism stage.
(2) The reduction of hematite comprises three steps, whereas that of CF comprises two steps. The reduction of powdered hematite and CF can be described by shrinking layer model, not shrinking core model completely.
(3) The activation energy of hematite reduction reaches 5.81 kJ·mol−1 on the basis of the model-free method, whereas the activation energies of CF reduction are 46.89 kJ·mol−1 and 43.74 kJ·mol−1 on the basis of the model-free method and the Arrhenius equation method, respectively.
The authors are grateful to the financial support of the Natural Science Foundation of China (Nos. 51104192 and 51544203).
1) α: reduction degree [–]
2) ∆mt and ∆m0: removed oxygen mass at a fixed time t and the theoretically removed oxygen mass from iron oxide [mg]
3) dα/dt: reduction rate [min−1]
4) k(T): rate constant [min−1]
5) f(α) and G(α): model function
6) E: activation energy [kJ/mol]
7) A: pre-exponent [min−1]
8) R: gas constant, 8.314 [J·(mol·K)−1]
9) n: Avrami exponent [–]
10) y(α): a defined non-dimensional parameter [–]