2016 Volume 56 Issue 12 Pages 2267-2275
2016 Volume 56 Issue 12 Pages 2267-2275
The effect of Mg3(PO4)2 and SrCrO4 pigments in paint layer on the corrosion behavior of 55 mass% Al–Zn coating at the cut edges and the delamination mechanism of the organic paint at cross-cut areas of pre-painted galvalume steels were examined using wet/dry cycle corrosion tests. The Mg3(PO4)2 pigment did not inhibit white rust formation at the cut edges and the delamination of the organic paint. On the other hand, the SrCrO4 pigment suppressed white rust formation and improved the delamination resistance of the organic paint. Observations of the cross sections of the cross-cut area indicate that delamination was the result of the formation of corrosion products in the Al–Zn layer under the organic paint layer, thus the SrCrO4 pigment inhibited the delamination of the organic paint layer by reducing the corrosion rate of the Al–Zn layer. The Kelvin probe force microscopic measurements revealed that while the addition of the anti-corrosive pigments increased the corrosion potential of the Al–Zn layers, it did not affect the electrochemical properties of the steel substrates. The increases in the corrosion potential can be attributed to the barrier effect of the corrosion products containing chromate pigments on the Al–Zn layer against anodic dissolution. From these results, it is reasonable that chromate pigments effectively increase the delamination resistance of organic paint layers on pre-painted galvalume steels under atmospheric corrosion conditions. In addition, phosphate pigments would improve the corrosion resistance of Al–Zn layers in atmospheric environments with low chloride concentrations.
Pre-painted hot-dip 55 mass% Al–Zn alloy coated (galvalume) steels are widely used as architectural and automotive materials due to their high weatherability in atmospheric environments. The high durability of pre-painted galvalume steels is derived from the sacrificial protection of steel by the Al–Zn coating layer, and anti-corrosive pigments are added to the primer layers to improve the delamination of the organic paint. Strontium chromate (SrCrO4) is typical of such anti-corrosive pigments, and effective against filiform corrosion on pre-painted steels at cut edges and coating defects.1,2,3,4) However, due to concerns regarding the toxicity of chromium (VI)-containing compounds, the use of SrCrO4 has been restricted,5) and the search for an alternative to chromate base pigments has been extensive.1,6,7,8,9,10,11,12,13,14)
Phosphate pigments are traditional and promising alternatives to chromate based pigments. Zinc phosphate (Zn3(PO4)2) is applied to a conversion treatment for the improvement of adhesion between organic paint layers and steels.15,16) Because of the high barrier performance of phosphate conversion coatings on steel substrates,17) it is widely believed that phosphate pigment also functions as a barrier type inhibitor against the dissolution of the Al–Zn layer for pre-painted galvalume steels. Some researchers have focused on the effect of phosphate pigments on the corrosion resistance of Zn-coated steels.18,19,20,21,22,23) Simões et al. studied the corrosion inhibition mechanism of sodium phosphate (Na3PO4) at the cut edges of electrogalvanized steels using scanning vibration electrode technique (SVET) and electrochemical impedance spectroscopy (EIS) in phosphate-containing 0.1 M NaCl solution. They summarized that Zn3(PO4)2, which was formed by the reaction between phosphate and Zn ions, inhibited the anodic dissolution of Zn;18,19) however, the protective performance of Zn3(PO4)2 was lower than that of zinc chromate.20) Zin et al. reported that the deposition of compact calcium/zinc phosphate species on the Zn coating layer and the steel substrate highly improved the efficiency of the inhibition effect of phosphates on cut-edge corrosion.21,22,23) These studies were carried out in immersion conditions in aqueous solutions. As such, the corrosion protection mechanisms of phosphate pigments on pre-painted steels in atmospheric environments remain unclear.
Because a better understanding of the role of chromates in the corrosion inhibition supports the development of alternatives, the effect of chromates on the cut-edge corrosion has been investigated widely. Chromate pigments are electrochemically reduced in aqueous solutions and are deposited as Cr(III) oxyhydroxide on the metal surface.24,25,26) The chromate is believed to affect the galvanic reaction between the Al–Zn coating layer and the steel substrate. Apparently, the delamination resistance of the paint layer is improved by chromate through the galvanic reaction at cut edges and cross-cut area.3,4,23) It should be noted, however, that a detailed understanding of the mechanism has yet to be presented. The purpose of this study is to elucidate the corrosion protection mechanism of phosphate and chromate in the primer layer of pre-painted hot-dip 55 mass% Al–Zn alloy coated steel. We focused on the effect of these anti-corrosive pigments on the electrochemical properties of the Al–Zn layer and the steel substrate, and also investigated the delamination mechanism of the organic paint layer under atmospheric corrosion conditions.
Kelvin probe force microscopy (KPFM) is a powerful tool in the analysis of corrosion reactions under thin electrolyte layers in atmospheric corrosion conditions. KPFM, which is a kind of scanning probe microscopy, can obtain the Volta potential difference (Kelvin potential) by measuring the electrostatic interaction between a KPFM probe and a specimen surface. Schmutz et al. demonstrated the corrosion potentials for pure metals in a chloride-containing solution and their Kelvin potentials in the air after the immersion followed a linear relationship,27,28) suggesting it is possible to estimate the corrosion potential distribution of metals under galvanic coupling from the Kelvin potential map immediately after corrosion tests. In this study, magnesium phosphate (Mg3(PO4)2) and SrCrO4 were selected as the anti-corrosive pigments. This is because Mg3(PO4)2 pigment is useful for corrosion inhibition of ferrous metals29,30) and SrCrO4 is well known as a typical anti-corrosive pigment. Wet/dry cycle tests were conducted to clarify the effect of the anti-corrosive pigments on the cut-edge corrosion behavior and the delamination of the organic paint. The Kelvin potential distribution at the interface between the Al–Zn coating layer and the steel substrate in the cut-edge specimen under atmospheric corrosion conditions was measured by KPFM. Polarization curves of the Al–Zn coating layers covered with corrosion products were taken to elucidate the role of the anti-corrosive pigments in cut-edge corrosion and the delamination of the organic paint.
Pre-painted hot-dip 55 mass% Al–Zn alloy coated (galvalume) steel sheets with and without anti-corrosive pigments were used as specimens. Either Mg3(PO4)2 or SrCrO4 was added to the primer layer as the anti-corrosive pigment. A galvalume steel sheet (0.6 mm in thickness) with approximately 20 μm of 55 mass% Al–Zn alloy layers on both sides was used as the starting material. Prior to painting, no conversion treatment was performed on the Al–Zn surface. The front of the galvalume steels was painted with a polyester primer ca. 5 μm in thickness with and without the anti-corrosive pigments. The concentration of the anti-corrosive pigments in the primer layer was adjusted to 20 mass%. Polyester topcoat layers of 5–10 μm in thickness were then formed on both sides of the sheet.
2.2. Wet/dry Cycle Test for Cut Edge CorrosionThe International Organization for Standardization (ISO) 16539 Method A was performed to simulate the initial stage of atmospheric corrosion at the cut edges of the specimens. Figure 1 shows the change in temperature and relative humidity (RH) as a function of time in a wet/dry cycle of ISO 16539 Method A. This is the simplified pattern derived from the variations of temperature and RH on a steel sheet exposed to an outdoor environment.31) The dew point of water vapor in the air was kept at 301 K to reproduce the summer season in Japan. It was demonstrated that the atmospheric corrosion behavior for pre-painted galvanized and galvalume steels can be reproduced using this corrosion test.3,4) An ESPEC SH221 programmable humidified chamber was used for the corrosion test.

Change in temperature and relative humidity (RH) as a function of time in the wet/dry cycle test (ISO 16539 Method A).
Figure 2(a) shows the model cut-edge specimen used for this corrosion test for the wet/dry cycle test. The pre-painted galvalume steels were cut into 10 mm × 15 mm squares, and the back side of the steels was polished to remove the Al–Zn layer and paint layers. After being polished, the specimen was embedded in an epoxy resin as shown in Fig. 2(a), and the cross section of the pre-painted galvalume steel was polished with 1500 grit SiC paper. The surface was covered with a polyimide tape except for the cut-edge region (5 mm × 15 mm). A thin electrolyte layer of 500 μm thickness was formed on the cut-edge region at the beginning of the corrosion test. The amount of Cl− ion deposition on the specimen was set to 0.1 g m−2 using 5.64 mM NaCl solution to simulate marine environments. All the solutions in this study were prepared from deionized water and analytical grade chemicals. Before and after 1 cycle of the wet/dry cycle test, the surface appearance of the cut edges was photographed by a digital camera.
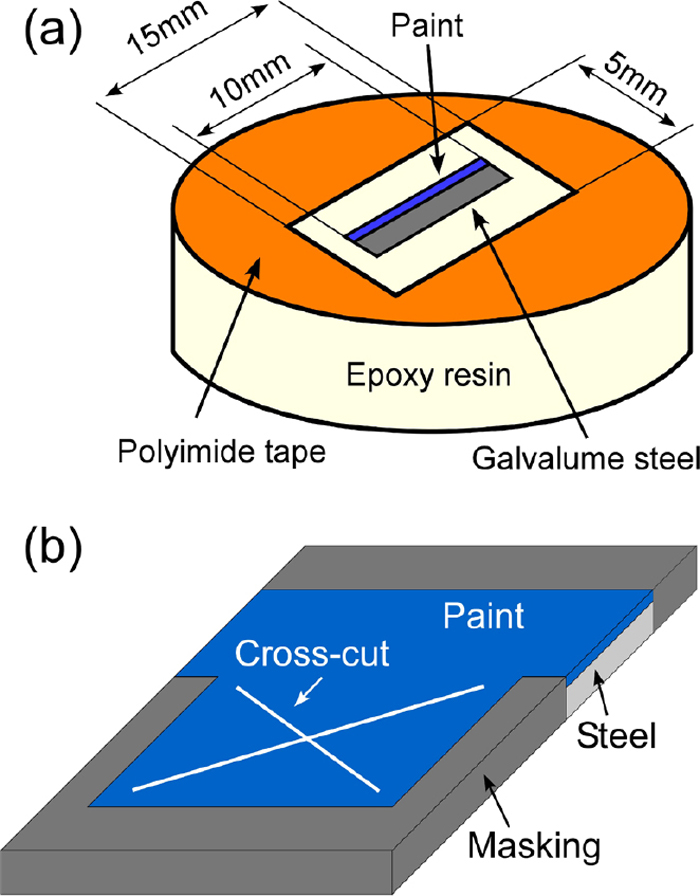
(a) Model cut-edge specimen used in the wet/dry cycle test (ISO 16539 Method A). (b) Cross-cut specimen used in the wet/dry cycle test (JASO M 609-91). (Online version in color.)
The Japanese Automobile Standards Organization (JASO) M 609-91, which is a cyclic corrosion test with salt spraying, was carried out to evaluate the effect of the anti-corrosive pigments on delamination of the organic paints at the cross-cut area. The salt water for JASO M 609-91 was 0.856 M NaCl. Because the chloride concentration of the salt water for JASO M 609-91 was higher than that for ISO 16539 Method A, JASO M 609-91 was suitable to evaluate the delamination of the organic paints efficiently. Figure 2(b) shows the cross-cut specimen used for this wet/dry test of JASO M 609-91. The cross-cut was made with a mechanical device and a folded back. The cyclic conditions of the JASO M 609-91 were as follows: 1) salt water spraying for 2 hours; 2) drying at 333 K and 20–30% RH for 4 hours; 3) wetting at 323 K and 95% RH for 2 hours. The wet/dry cycle was conducted for 60 times.
2.4. Kelvin Probe Force MicroscopyKelvin probe force microscopy (KPFM) was carried out using a Bruker Multimode 8 to measure the potential distribution of the Al–Zn coating/steel interfaces. The pre-painted galvalume steels were cut into 8 mm × 15 mm squares, and the back side of the steel sheet was polished to remove the Al–Zn and paint layers. After that, the steel sheets were embedded in an epoxy resin, and then the cross-section of the pre-painted galvalume steel was polished with diamond paste down to 1 μm. Except for the cut-edge region (5 mm × 5 mm), the specimen surface was covered with polyimide tape. In order to simulate the initial stage of the sacrificial protection, the specimens were corroded under a thin electrolyte layer at 301 K and 90% RH for 1 hour in the humidified chamber. Because KPFM were not suitable for the measurements of the specimen with many corrosion products, 5.64 mM NaCl was used to form an electrolyte layer 500 μm in thickness. After the corrosion test, KPFM measurements of the specimen were carried out immediately without any water-rinse treatment. The tip used was a Pt–Ir coated and electrically conductive Si probe (Bruker, SCM-PIT), with tip radius, resonant frequency, and spring constant of 20–25 nm, 75 kHz, and 2.8 N m−1, respectively. The topographical images were obtained by atomic force microscopy (AFM) simultaneously with the potential distributions by KPFM. The scan rate of the tips during the KPFM and AFM measurements was 0.2 Hz.
2.5. Polarization MeasurementsIn order to discuss the Kelvin potential of the Al–Zn coated layers in terms of the effect of the anti-corrosive pigments, both anodic and cathodic polarization curves were measured. Galvalume steel sheet without a paint layer, which was the starting material for pre-painting, was corroded for 3 cycles by ISO 16539 Method A. A thin electrolyte layer was formed using 5.64 mM NaCl, 5.64 mM NaCl–5.64 mM SrCrO4, or 5.64 mM NaCl–9.88 μM Mg3(PO4)2 solution. In this case, 9.88 μM Mg3(PO4)2 was the saturated concentration of Mg3(PO4)2 in water at 298 K. The anti-corrosive pigments were thought to be introduced into corrosion products, and their electrochemical properties were probably modified. The surface area of the working electrodes was 10 mm squares. 0.1 M NaCl at 298 K was used as the electrolyte in the polarization measurements. While the chloride concentration of the electrolyte for the polarization curves was higher than that for the KPFM measurements, 0.1 M NaCl is a commonly used for evaluation of the corrosion property in chloride-containing environments. The reference electrode was an Ag/AgCl (3.33 M KCl) electrode, and the counter electrode was a Pt sheet. The anodic and cathodic polarization measurements were performed in deaerated and air-saturated solutions, respectively. The scan rate of the electrode potential was 3.8 × 10−4 V s−1 (23 mV min−1).
2.6. Surface and Cross Section ObservationsThe surface appearances of the specimens before and after the wet/dry corrosion tests were taken by a Nikon D1 digital camera. Cross sections of the pre-painted galvalume steels were observed by a JEOL JSM-7100F scanning electron microscope (SEM) equipped with a JEOL JED-2300 energy-dispersive X-ray spectrometer (EDS). Prior to the SEM observation, the cross-section of the specimens were prepared by a JEOL IB-09010CP cross section polisher (Ar+ ion milling), and a Au thin film was deposited on the observed areas to avoid the charge up of the specimens.
To confirm the particle size and distribution morphology of the anti-corrosive pigments in the primer layer, SEM/EDS analyses were performed. Figures 3(a) and 3(b) show the cross-sectional SEM images of the specimens with the Mg3(PO4)2 and SrCrO4 pigments. The large white (pale gray) particles observed in the primer layer were thought to be the anti-corrosive pigments. Figure 3(c) indicates the comparison of the EDS spectra obtained from inside (Point A1) and outside (Point A2) of the particle in the primer layer with Mg3(PO4)2 pigment. The intensities of Mg, P, and O at Point A1 were higher than those at Point A2, suggesting that the large particles in the primer layers corresponded to the Mg3(PO4)2 pigment. Also in the specimen with the SrCrO4 pigment (Fig. 3(d)), the intensities of Sr, Cr, and O inside the particle (Point B1) were higher than those outside the particle (Point B2), implying that the large particles in the primer layers corresponded to the SrCrO4 pigments. As seen in Figs. 3(a) and 3(b), because pigments are normally produced by milling processes,32) the pigment particles were coarsely distributed over the range from 0.5 to 5 μm.

(a, b) Cross-sectional SEM images of the specimens with Mg3(PO4)2 or SrCrO4 pigment in the primer layers. (c, d) EDS spectra obtained from inside (Points A1 and B1) and outside (Points A2 and B2) the particles in the primer layers shown in (a) and (b).
In addition to the above characteristics of the distribution of the anti-corrosive pigments, the microstructure of 55% Al–Zn layer was confirmed to be dual-phase. It is well known that the primary solidification phases are Al-rich with a granular-shape, and that Zn-rich phases exist at the intergranular region. In Figs. 3(a), 3(b), the white (pale gray) areas along grain boundaries were likely to be the Zn-rich phases.
3.2. White Rust Formation at Cut EdgesThe wet/dry cyclic corrosion test (ISO 16539 Method A) was performed to investigate the effect of Mg3(PO4)2 and SrCrO4 pigments on the corrosion of the Al–Zn layer at the cut edges. Figure 4 shows the surface appearances of the model cut-edge specimens with and without the anti-corrosive pigments after 1 cycle. The Al–Zn coating and the organic paint layers are located in the lower side of the images shown in Fig. 4. It was clearly observed that less white rust formed on the SrCrO4-containg specimen than on the Mg3(PO4)2-containing and the pigment-free specimens. The addition of SrCrO4 was confirmed to prevent the Zn dissolution at cut edges, but Mg3(PO4)2 was thought to be relatively ineffective. The results obtained in this study are consistent with those in the literature.3,4,21)
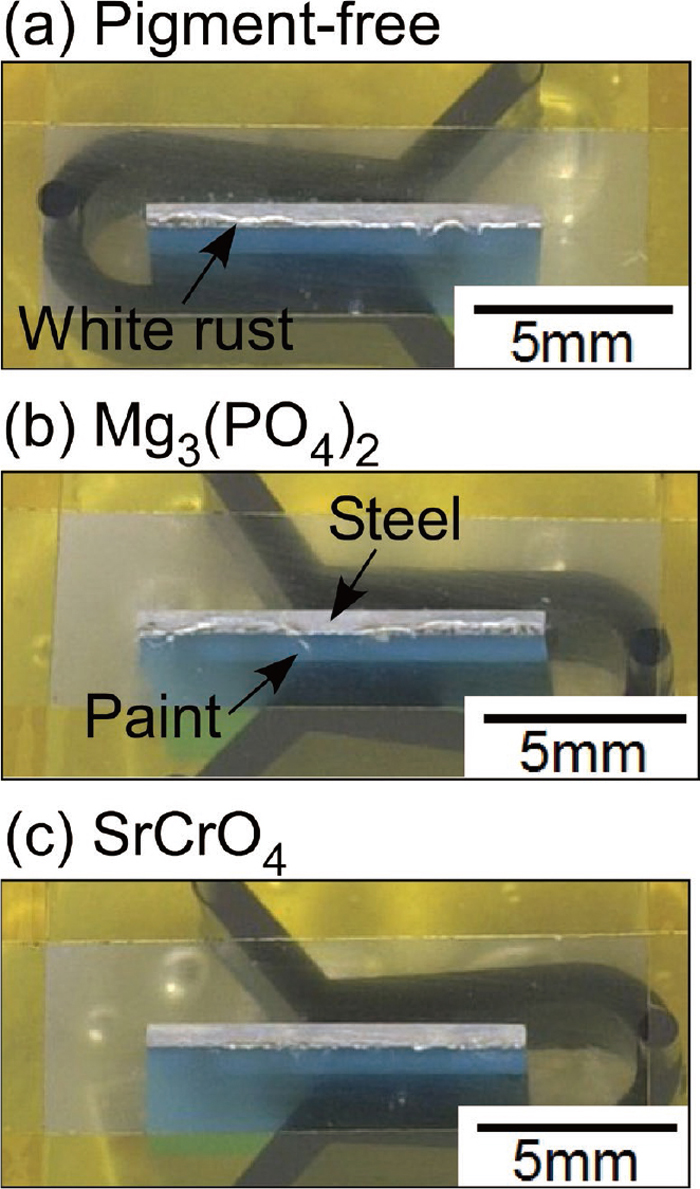
Surface appearances of the model cut-edge specimens with and without the anti-corrosive pigments in the primer layer after 1 cycle of the wet/dry corrosion test (ISO 16539 Method A).
The cyclic wet/dry test (JASO M 609-91) was carried out to ascertain the effect of Mg3(PO4)2 and SrCrO4 pigments on the delamination resistance of organic paint layers. Figure 5 shows the maximum delamination length of the paint layers from the cross-cut on the pre-painted galvalume steels with and without the anti-corrosive pigments after 60 cycles in the wet/dry cycle test. The delamination resistance of the organic paint layers at the cross-cut on the specimen was improved by the addition of SrCrO4 pigment. On the other hand, the delamination length of the paint layer with the Mg3(PO4)2 pigment was comparable to that without the anti-corrosive pigments. From the result of the cyclic wet/dry corrosion tests (ISO 16539 Method A and JASO M 609-91), it appears that the Mg3(PO4)2 pigment had little effect on the corrosion resistance of the Al–Zn layer at cut edges and the delamination resistance of the organic paint layers.

Maximum delamination length of the organic paint from the cross-cut on the pre-painted galvalume steels with and without anti-corrosive pigments after 60 cycles of the wet/dry (JASO M 609-91).
The cross-cut areas of the specimens after the cyclic wet/dry test (JASO M 609-91) were observed by SEM to clarify the delamination mechanisms of the organic paint layer. To avoid chemical species dissolving in water by wet-polishing, the cross section polisher (Ar+ ion milling) was employed in the preparation of the cross-sections. Figures 6(a)–6(c) show the cross-sectional SEM images and EDS maps in the vicinity of the cross-cuts on the pre-painted galvalume steels with and without the anti-corrosive pigments. The delaminated areas were marked by the horizontal thick arrows in Figs. 6(a)–6(c). In the case of the specimen without the anti-corrosive pigments (Fig. 6(a)), signals of Cl and O were found to co-exist at the region between the organic paint and Al–Zn layers (marked by C1). In this co-existence region, signals of Al and Zn were detected. It was likely that the corrosion products of Al and Zn were generated under the paint layer by the migration of Cl− ions in the wet/dry cycle test, which caused the delamination of the organic paint on the Al–Zn layer. In addition to this, a characteristic feature of corrosion morphology was observed in the Al–Zn layer. The co-existence region of Cl and O signals was extended into the Al–Zn layer (marked by C2). The intergranular Zn-rich phases were thought to corrode preferentially under the wet/dry conditions, and the corrosion products of Zn, such as ZnCl2, Zn(OH)2, Zn5(CO3)2(OH)6, Zn5(OH)8Cl2·H2O etc. were formed in the grain boundaries in the Al–Zn alloy layer.33) These results show that volume expansion due to the formation of corrosion products of Zn and Al between the organic paint and the coating layers was likely to be the main factor of the delamination of the organic paint layer.
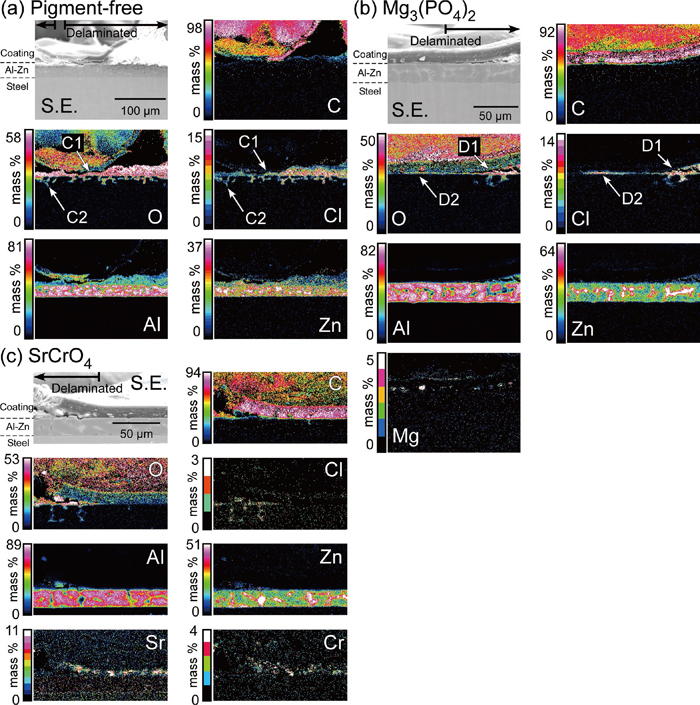
Cross-sectional SEM images and EDS maps in the vicinity of the cross-cuts on the pre-painted galvalume steels after the cyclic wet/dry test of JASO M 609-91: (a) specimen without the anti-corrosive pigment, (b) with Mg3(PO4)2 pigment, and (c) with SrCrO4 pigment in the primer layer.
Figure 6(b) shows the EDS maps of the specimen with the Mg3(PO4)2-containing organic paint layer after the wet/dry cycle test. Also in this case, signals of Cl and O co-existed, and that of Mg was not detected in the delaminated region (marked by D1). Moreover, even in the un-delaminated (intact) region, Cl and O signals were detected (marked by D2), implying that the formation of the corrosion products of the Al–Zn layer caused the delamination of the organic paint in the same way as the pigment-free specimen. According to this mechanism, the corrosion rate of the Al–Zn layer is the predominant factor affecting the delamination resistance of the paint layers. As shown in Fig. 4, almost the same amount of white rust formed at the cut edge on the specimen with the Mg3(PO4)2 pigment as on the specimen without the anti-corrosive pigments, which explains the similarity in the delamination length of the specimen with the Mg3(PO4)2 pigment and that of the specimen without the anti-corrosive pigments, as shown in Fig. 5.
Figure 6(c) exhibits the EDS maps of the specimen with the SrCrO4-containing organic paint layer after the wet/dry cycle test. In this case, the signals of O and Cl co-existed in the delaminated region, and Sr and Cr did not exist at the same region. This suggests that the specimen with the SrCrO4-containing organic paint followed the same delamination mechanism as that mentioned above. However, extremely low amounts of Cl and O were found for the specimen with SrCrO4-containing pigment compared to those with the Mg3(PO4)2 pigment and without the anti-corrosive pigments. The results shown in Fig. 4 indicate that the addition of SrCrO4 pigment was effective for the suppression of the corrosion of the Al–Zn layer. Suppressing the dissolution of the Al–Zn layer by anti-corrosive pigments is concluded to be one of the key points in the improvement of the delamination resistance of organic paints on galvalume steels.
3.4. Potential Distribution at Al–Zn Layer/steel InterfacesAs mentioned above, the dissolution of the Al–Zn layer results in the delamination of the organic paints, and hence the effect of anti-corrosive pigments on the Al–Zn layers provides important information regarding the corrosion protection mechanisms of the anti-corrosive pigments. Kelvin potential distributions at the Al–Zn layer/steel substrate interface were measured by the KPFM. Since the KPFM measurements were conducted immediately after the corrosion test at 301 K and 90% RH for 1 hour, the measured Kelvin potentials are closely related to the corrosion potential on the specimen surfaces.27,28) The Kelvin potential (Vk) measured by KPFM is referred to the Pt–Ir tip in this study; −Vk is proportional to the corrosion potential.
Figure 7 shows the AFM height and KPFM potential images at the Al–Zn layer/steel interface of the pigment-free specimen. From the AFM height image, it was confirmed that the dissolution of the Al–Zn layer in the vicinity of the steel was relatively high, and the corrosion products were deposited on the steel substrate and the Al–Zn layer. More corrosion products were deposited on the steel substrate than on the Al–Zn layer, suggesting that corrosion products were mainly deposited along with the oxygen reduction reaction on the steel substrate. It was confirmed that the galvanic coupling between the Al–Zn layer and the steel substrate takes place under a thin electrolyte layer in the atmospheric corrosion test, which was performed prior to KPFM measurements. The Kelvin potential of the steel substrate was lower than that of the Al–Zn layer, indicating that the corrosion potential of the steel substrate was higher than that of the Al–Zn layer.
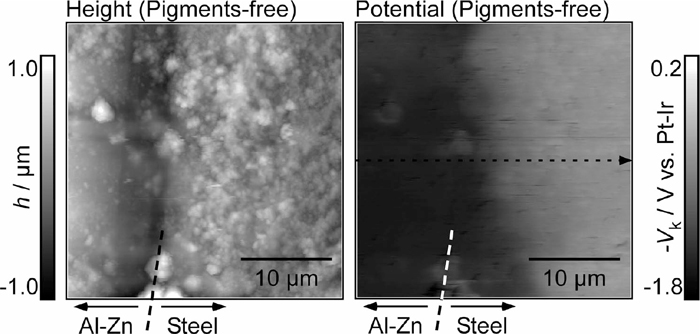
AFM height image and corresponding Kelvin potential map at the Al–Zn layer/steel interface of the pigment-free specimen after the corrosion test for 1 h.
Figure 8 exhibits the AFM height and KPFM potential images at the Al–Zn layer/steel interface of the specimen with the Mg3(PO4)2 pigment. As was the case for the pigment-free specimen, the Al–Zn layer dissolved to a depth of ca. 2 μm, and the finer corrosion products were deposited on the steel substrate (marked by E1). The large particles were thought to be NaCl from the electrolyte. A smaller Kelvin potential difference was noted between the Al–Zn layer and the steel of the specimen with the Mg3(PO4)2 pigment than that of the pigment-free specimen. In particular, it appeared that the Kelvin potential of the Al–Zn layer for the specimen with the Mg3(PO4)2 pigment was lower than that of the specimen without the anti-corrosive pigments. On the other hand, the Kelvin potential of the steel substrate was almost the same as that of the specimen without the anti-corrosive pigments.
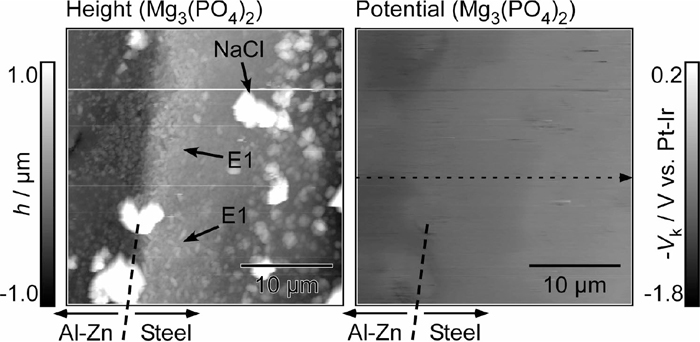
AFM height image and corresponding Kelvin potential map at the Al–Zn layer/steel interface of the specimen with Mg3(PO4)2 pigment in the primer layer after the corrosion test for 1 h.
Figure 9 shows the AFM height and KPFM potential images at the Al–Zn layer/steel interface of the specimen with the SrCrO4 pigment. From the AFM height image, neither the dissolution of the Al–Zn layer nor the deposition of corrosion products was severe in this case. This result corresponds to the inhibition of the white rust formation at the cut edge shown in Fig. 4. The Kelvin potential difference between the Al–Zn layer and the steel of the specimen with the SrCrO4 pigment was also smaller than that of the pigment-free specimen.
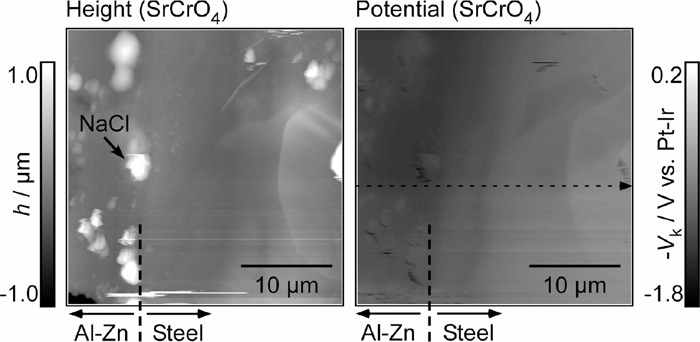
AFM height image and corresponding Kelvin potential map at the Al–Zn layer/steel interface of the specimen with SrCrO4 pigment in the primer layer after the corrosion test for 1 h.
The line profiles of the Kelvin potentials along the black arrows shown in Figs. 7, 8, 9 are summarized in Fig. 10. The horizontal axis is the distance apart from the left sides of the images of Figs. 7, 8, 9. From Fig. 10, it is seen that the Mg3(PO4)2 and SrCrO4 pigments increased the corrosion potentials of the Al–Zn layers. On the other hand, the corrosion potentials of the steel substrates were almost the same; that is, the anti-corrosive pigments did not affect the potentials of the steel substrates. In all the known earlier studies, the protection mechanism of anti-corrosive pigments against the corrosion of galvanized and galvalume steels has been studied in aqueous solutions. In phosphate or chromate containing solutions, a deposition layer composed of phosphates or Cr(III) oxyhydroxides was probably formed on steel substrate, inhibiting the oxygen reduction reaction.4,18,21,26) However, the KPFM results of this study indicate that the corrosion potential of the steel substrates was not significantly influenced by the anti-corrosive pigments; as such, the pigments themselves appear to act as anodic inhibitors that suppress the dissolution rate of the Al–Zn layer.

It is likely that the anti-corrosive pigments were introduced into the corrosion products of the Al–Zn layer, and that their electrochemical properties were modified. In order to confirm that the anti-corrosive pigments act as the anodic inhibitor of the Al–Zn layer, the anodic and cathodic polarization curves of the 55 mass% Al–Zn layer (galvalume) covered by a white rust layer were measured in 0.1 M NaCl solution. The galvalume steel steels were corroded under the thin electrolyte layers with and without the anti-corrosive pigments, and then the corroded surface of the Al–Zn layer was used as a working electrode. Figure 11 shows the experimental results of the polarization behavior. As seen in this figure, there was little or no effect of the anti-corrosive pigments on cathodic polarization behavior. On the other hand, the anodic polarization curves of the Al–Zn alloy layer covered with the white rust formed in the SrCrO4-containing electrolyte layer differed from those obtained from the specimens corroded in the electrolytes with the Mg3(PO4)2 and without the pigments. It is clear that the white rust generated in the SrCrO4-containing electrolyte suppressed the anodic dissolution rate of the Al–Zn layer. The addition of SrCrO4 pigment to the primer layer is thought to increase the corrosion potential of the Al–Zn layer; however, the corrosion potential of the Al–Zn layer remained lower than that of the steel substrate. As a result, the Al–Zn layer can offer a sacrificial corrosion protection effect to the steel substrate. Furthermore, because the dissolution rate of Al–Zn layer is suppressed, the sacrificial corrosion protection effect of the Al–Zn layer continues for a long period of time even under thin electrolyte layers. This explains how the SrCrO4 pigment in the primer layer inhibited the white rust formation at cut-edge specimen and the subsequent delamination of the organic paint layer, as shown in Figs. 4 and 5.
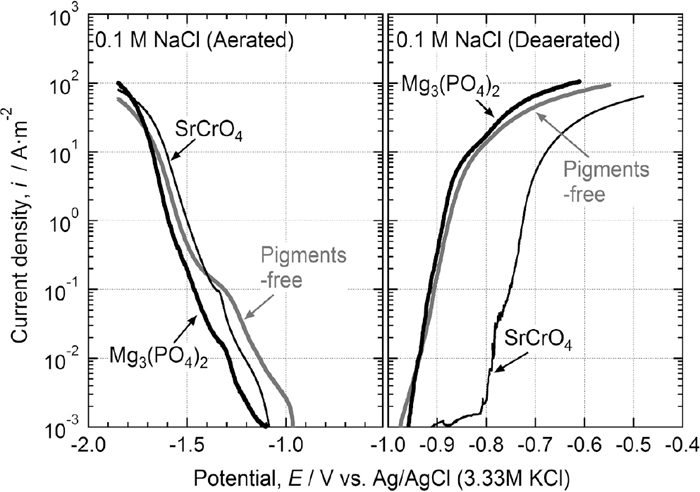
Anodic and cathodic polarization curves for the galvalume steel surfaces covered with white rust layer formed in wet/dry cycle test (ISO 16539 Method A) for 3 cycles. Corrosion tests were performed under a thin electrolyte layer with and without anti-corrosive pigments (Mg3(PO4)2 and SrCrO4). The anodic and cathodic polarization measurements were carried out in 0.1 M NaCl at 298 K under deaerated and air-saturated conditions, respectively.
As shown in Fig. 11, the anodic polarization behavior of the rust layer formed in the Mg3(PO4)2-containing solution was similar to that in the pigment-free solution. The Mg3(PO4)2 pigment does not improve the protective ability of the corrosion products of the Al–Zn layer in 0.1 M NaCl. Simões et al. reported that a Zn3(PO4)2 film formed on the Zn layer was locally destroyed in a chloride-containing solution, and the anodic dissolution of Zn occurred even when the Zn surface was covered by the corrosion products.18) It was concluded that the anodic dissolution of the Al–Zn was not inhibited due to the local breakdown of the layer of the corrosion products on the Al–Zn surface in the polarization measurements. The polarization measurements were performed in 0.1 M NaCl solution, and the cyclic wet/dry test (JASO M 609-91) was carried out using 0.856 M NaCl solution as the salt water. On the other hand, because the KPFM measurements were conducted after the corrosion test under the electrolyte layer of 5.64 mM NaCl solution, the Mg3(PO4)2 pigment was thought to increase the corrosion potential of the Al–Zn layer. It was expected that the Mg3(PO4)2 pigment addition in the primer layer would improve the atmospheric corrosion resistance of the pre-painted galvalume steels in a specific environment with low chloride concentrations.
(1) Less white rust formed on the pre-painted 55 mass% Al–Zn alloy coated (galvalume) steel with the SrCrO4 pigment at the cut edge than on those with the Mg3(PO4)2 and without anti-corrosive pigments in the wet/dry cyclic corrosion test (ISO 16539 Method A). In addition, less delamination was noted in the organic paint layer of the SrCrO4-containing cross-cut specimen than in that of the Mg3(PO4)2-containing and the pigment-free specimens in the wet/dry cyclic corrosion test (JASO M 609-91).
(2) The delamination of the organic paint layers for the pre-painted galvalume steels can be attributed to the formation of the corrosion products of Al and Zn between the organic paint and the Al–Zn coating layer. The SrCrO4 pigment inhibited the delamination of the organic paint layer by reducing the corrosion rate of the Al–Zn layer.
(3) The Mg3(PO4)2 and SrCrO4 pigments increased the corrosion potentials of the Al–Zn coated layers, and did not affect the potentials of the steel substrates. This indicates the Mg3(PO4)2 and SrCrO4 pigments act as anodic inhibitors.
(4) Chromate pigments reduce the corrosion rate of Al–Zn layers, and increase their corrosion potential. However, because the corrosion potential of Al–Zn layers remains lower than that of steel substrates, the duration of the sacrificial protection by Al–Zn coated layer for anti-corrosive pigments-containing specimens is longer than that for pigments-free specimens. It is expected that chromate pigments effectively improve the delamination resistance of organic paint layers for pre-painted galvalume steels under atmospheric corrosion conditions.
(5) The anodic polarization curves in 0.1 M NaCl showed that the corrosion products generated in the Mg3(PO4)2-containing electrolyte did not suppress the anodic dissolution of the Al–Zn layer. However, because the Mg3(PO4)2 pigment increased the corrosion potential of the Al–Zn layer, it is expected that phosphate pigments would enhance the corrosion resistance of pre-painted galvalume steels in atmospheric environments with low chloride concentrations.