2016 Volume 56 Issue 6 Pages 1023-1030
2016 Volume 56 Issue 6 Pages 1023-1030
The sulfide morphology in SUS303 was investigated and the mechanism of monotectic sulfide formation was evaluated. The sulfide of metastable monotectic type was observed at the surface region of ingot with the higher solidification cooling rate and stable eutectic type was observed at the inner region. The formation of monotectic sulfide was promoted by Ca addition and the formation of eutectic type sulfide was promoted by Al addition with the lower oxgen content. It is thought that the nucleation behavior greatly affect the monotectic sulfide formation because of the lower interfacial energy with liquid oxide inclusion. However, solute distribution, undercooling and the decrease of S activity by adding Ca or increasing O content may also influence on the eutectic/monotectic morphology selection.
SUS303 is an austenitic stainless steel containing more than 0.15% sulfur, which improves its machinability. The machinability of austenitic stainless steel is enhanced by an increase in the amount of sulfides1,2,3) and is also largely influenced by the distribution, morphology, and composition of the inclusions. For example, larger, more spherical sulfides are favorable for chip disposability.4) It has been also shown that control of inclusion morphology by the addition of Ca and Se improves tool wear and surface roughness.5,6)
In their study on the morphology of sulfide inclusions formed in steel during solidification, Oikawa et al.7) showed that manganese sulfide (MnS) is formed by the stable eutectic or metastable monotectic reaction, and the properties (e.g. solid/liquid or interfacial energy) of non-metallic inclusions, which become nucleation sites, contribute greatly to the type of sulfide morphology developed. Additionally, for SUS303, hot workability was improved by adjusting the Ca and O contents because formation of eutectic sulfides was limited and granular monotectic sulfides were formed instead.8) Furthermore, observation using a confocal scanning laser microscope revealed that granular sulfides, which seemed to be monotectic, were formed in free-machining steel solidified through primary δ solidification.9) However, it is thought that in free-machining austenitic stainless steel, such as SUS303, the γ phase is formed during solidification after formation of the primary δ phase, and then, the sulfides precipitate. This phenomenon is complicated and the mechanism of the change in sulfide morphology has not been elucidated.
In the case of free-machining steel, uniform distribution of sulfides with low aspect ratio is important for improving properties such as machinability10,11) and the first task is to clarify how the selection between monotectic and eutectic occurs, which forms the basics of sulfide morphology control. Therefore, we investigated the effect of additional elements on sulfide morphology using cast SUS303 and examined the factors causing monotectic or eutectic selection.
Table 1 shows the chemical composition of the specimens used in this study. SUS303 (No. 1) is used as a base material, and Al (No. 2) or Ca (Nos. 3 and 4) was added to SUS303. All specimens were obtained from the middle part of the ingots cast in a 16-kg vacuum induction-melting furnace. The target tapping temperature was 1560°C (superheating degree was set to 100°C) and casting was performed over a range of 10°C in either direction of the target temperature.
| (mass%) | |||||||||||||
| C | Si | Mn | P | S | Ni | Cr | Mo | Cu | Al | Ca | T.O | N | |
| 1 | 0.062 | 0.35 | 1.8 | 0.030 | 0.32 | 9.7 | 18.8 | 0.11 | 0.41 | 0.002 | 0.0001 | 0.011 | 0.037 |
| 2 | 0.061 | 0.35 | 1.8 | 0.030 | 0.32 | 9.7 | 18.8 | 0.11 | 0.41 | 0.029 | 0.0001 | 0.006 | 0.036 |
| 3 | 0.061 | 0.35 | 1.8 | 0.030 | 0.31 | 9.7 | 18.7 | 0.11 | 0.41 | 0.007 | 0.0036 | 0.015 | 0.036 |
| 4 | 0.062 | 0.35 | 1.8 | 0.030 | 0.32 | 9.7 | 18.7 | 0.11 | 0.41 | 0.008 | 0.0067 | 0.017 | 0.036 |
First, the sulfide morphology was observed from the surface layer to the inside of the ingot under an optical microscope. Next, in order to determine the solidification cooling rate, secondary dendrite arm spacing was measured after electrolytic etching with oxalic acid. The spacing of about 20 secondary arms was measured to obtain the average value, which had a deviation of about 20%. In addition, the morphology and composition of the inclusions (sulfides and oxides) in the metallographic samples and the extracted residues were examined by scanning electron microscopy equipped with energy dispersive X-ray spectrometry (SEM-EDS). The extracted residues were collected by electrolyzing a metallographic sample, which was obtained from 10–30 mm below the surface of the ingot, in maleic anhydride and filtered using a 0.2 μm mesh filter paper. For some of the samples, the distribution of chemical composition in the sulfides was investigated using an electron probe micro analyzer (EPMA).
2.2. Liquid-tin Quenching ExperimentFor the specimen with added Ca (No. 4), a 5 mm-thick sample was obtained from the longitudinal section (L-section) near the surface layer of the ingot and was used for a quenching experiment with liquid tin during gas tungsten arc (GTA) welding.12) The GTA welding conditions were 120A-10V-10 cm/min. Frozen microstructure evolution process was observed under an optical microscope and the primary dendrite arm spacing was measured after electrolytic etching with oxalic acid to estimate the solidification cooling rate. In addition, microsegregation near the solidification interface was investigated by EPMA.
Similar to past findings, the following two types of MnS were observed: (i) eutectic (consisting of fine rod-like MnS) and (ii) monotectic (relatively large granular MnS). Figure 1 shows the typical morphology of sulfides: optical micrographs of the cross sections of the sulfides and SEM images of the sulfides extracted by electrolysis. The chemical composition of the sulfides was (Mn,Cr)S, a main constituent was Mn (43–51 mass%). As shown in Table 2, there was no significant difference in composition between the eutectic and monotectic types, and they can be predicted in equilibrium calculation. The equilibrium calculation was performed by the computational thermodynamics software, PANDAT.13) It should be noted that although a trace amount of oxygen was detected in monotectic sulfides, it was neglected in this table for comparing the measured data with the calculated values. Figures 2 and 3 show the distribution of sulfides in the ingots. In the case of normal SUS303 (No. 1), only the surface layer (1 mm below the surface) was monotectic and the bulk was mainly eutectic. The entire region, from the surface layer to the inside, was eutectic for the sample with Al addition (No. 2). In the case of the samples with added Ca (Nos. 3 and 4), the monotectic type was present more deeply in ingot than the additive-free sample No. 1, and the region of the monotectic sulfides in sample No. 4, which contains more Ca, was broader than that of sample No. 3. In sample No. 4, the transition from monotectic to eutectic was found at 20–30 mm below the surface.

Eutectic and monotectic sulfides in SUS303 ingots. (a) No. 2, eutectic, optical micrograph, (b) No. 4, monotectic, optical micrograph, (c) No. 2, SEM image for extracted sulfides, (d) No. 4, SEM image for extracted sulfides.
| (mass%) | |||||
| S | Mn | Cr | Fe | ||
| EDS analysis in No. 1 | eutectic | 37.4 | 48.8 | 12.4 | 1.5 |
| monotectic | 37.8 | 47.5 | 13.8 | 0.9 | |
| Calculation at 1573 K | eutectic | 37.1 | 49.4 | 13.1 | 0.4 |
| monotectic | 37.0 | 47.8 | 14.0 | 1.2 | |

Distribution of sulfide in SUS303 ingots (No. 1, 2).
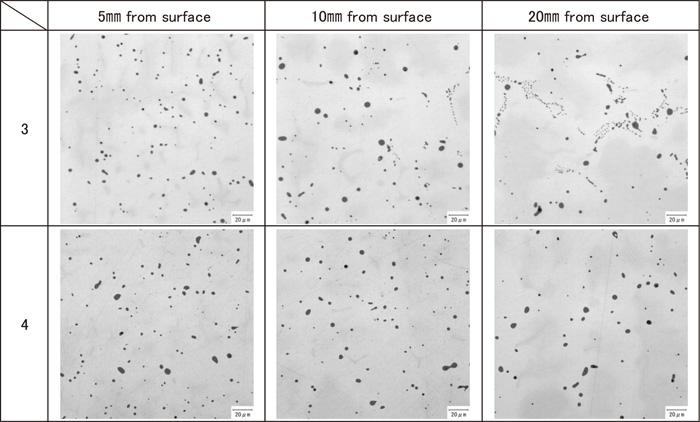
Distribution of sulfide in SUS303 ingots (No. 3, 4).
Figure 4 shows an estimated solidification cooling rate in the surface layer of ingot No. 1. After measurement of the secondary dendrite arm spacing, the solidification cooling rate was estimated using the equation reported by Mizukami et al.14) The cooling rate was faster closer to the surface of the ingot, and the cooling rate of the casting material used in this study was similar to that of a continuous cast billet. From Figs. 2, 3, 4, metastable monotectic sulfides were readily formed on the side close to the surface layer, where the solidification cooling rate and the solidification velocity were fast. In addition, monotectic sulfides were large at the inner part of ingot, where the dendrite arm spacing was large.
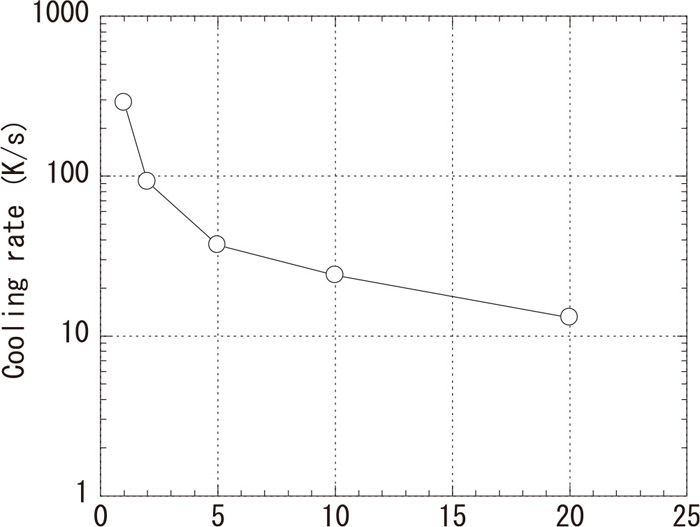
Estimated solidification cooling rate in SUS303 ingot (No. 1).
In the case of the sample with Ca addition, (Mn,Cr)S was partly formed around the CaO–SiO2–Al2O3 inclusions, and the surrounding sulfides contain a minute amount of Ca. Figure 5 shows the duplex inclusions in the sample with Ca (No. 4), observed by SEM-EDS. Formation of such duplex inclusions with a double structure was reported in the case of Ca-treated free-machining steel.15) Formation of calcium sulfide (CaS), which was formed in high sulfur steel,16) was not observed in this work.
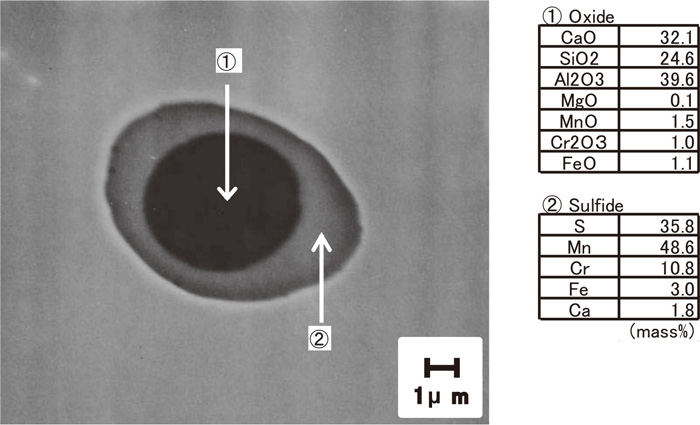
Morphology of inclusion in Ca added SUS303 (No. 4).
For sample No. 4, the microstructure and microsegregation near the solidification interface quenched by liquid tin were investigated, and the results are shown in Fig. 6. Near the quenched interface (Zone I), an increase in nickel (Ni) concentration was observed between the primary δ dendrite arms, but no segregation of Cr was found.
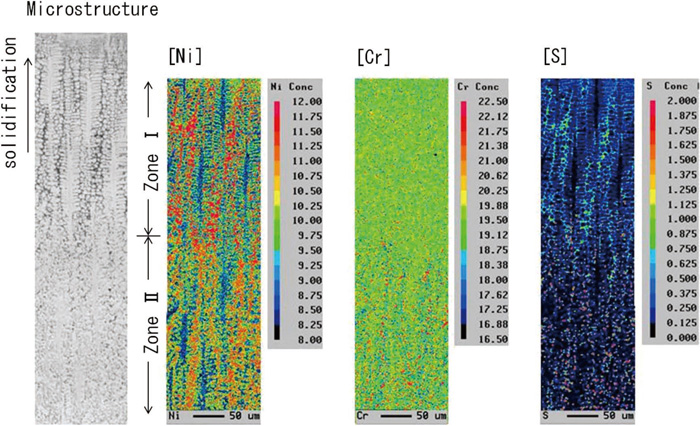
Microstructure and distribution of composition during weld solidification quenched by liquid tin.
In the area over 300 μm from the interface (Zone II), formation of relatively large sulfides and segregation of Cr were distinctly observed. In the primary δ solidification, although the distribution coefficient of Cr is close to 1 and solidification segregation does not occur, the change in Cr concentration becomes conspicuous because of δ/γ transformation. Therefore, γ phases and sulfides were thought to form in Zone II. Optical microscope observation revealed that sulfides at this region mainly take a granular form, like the casting experiment, and they were considered monotectic. The solidification cooling rate estimated from primary dendrite arm spacing and the equation of Mizukami et al.17) was about 100 K/s, which was similar to that of the surface layer of the casting material used in this work.
3.3. Phase Diagram and Sulfide FormationFigure 7(a) shows the calculation result of the equilibrium diagram of Fe-19.0%Cr-9.8%Ni-0.060%C-1.8%Mn-0.32%S- 0.035%N alloy using PANDAT. It should be noted that the components not described above were factored into either the Cr or Ni concentration by using a Cr or Ni equivalent.18) Stable eutectic sulfides are expected to form when the solid phase fraction is about 0.4. For the formation of sulfides, an increase in the concentration of S in the molten steel due to the formation of γ phases, which has a low solubility of S, is considered important.19) Figure 7(b) shows the calculation result of the formation of metastable monotectic sulfides from the same components without considering the stable eutectic sulfides. The difference in the precipitation temperatures (between Figs. 7(a) and 7(b)) is as small as about 8 K, and there is competition between the stable eutectic and the metastable monotectic.
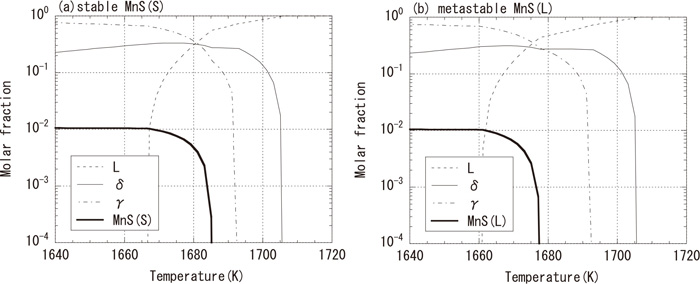
Calculated phase diagram in SUS303.
The oxide inclusions formed in normal SUS303 (such as sample No. 1), the sample with added Al, and the sample with added Ca, are SiO2–MnO–Cr2O3, Al2O3, and CaO–SiO2–Al2O3, respectively. As discussed by Oikawa et al.,7) it is considered that, by addition of Al or Ca to SUS303, solid oxide Al2O3 or liquid-phase oxide CaO–SiO2–Al2O3, respectively, was formed, which act as nucleation sites for the stable eutectic MnS(S) or the metastable monotectic MnS(L). Therefore, it is supposed that when the liquid-phase oxide was formed, metastable monotectic sulfides tend to form because nucleation is the controlling step.
3.4. Influence of Solidification Velocity and Cooling RateConsidering the fact that metastable monotectic sulfides were formed when the solidification velocity or the solidification cooling rate is large, as shown in Figs. 2 and 3, the kinetic factors affecting the selection of sulfide morphology should be also examined. Figure 8 shows the relationship between temperature and concentration of S in molten steel calculated by the Scheil solidification model using PANDAT. Components for calculation are the same as those shown in Fig. 7, and a solid line and a broken line in this figure correspond to eutectic and monotectic sulfide formation, respectively. Although the concentration of S in molten steel increases with microsegregation, it decreases with sulfide formation. Difference in the temperatures of sulfide formation between the eutectic and monotectic phase is about 8 K, which is comparable to the result of the equilibrium calculation.
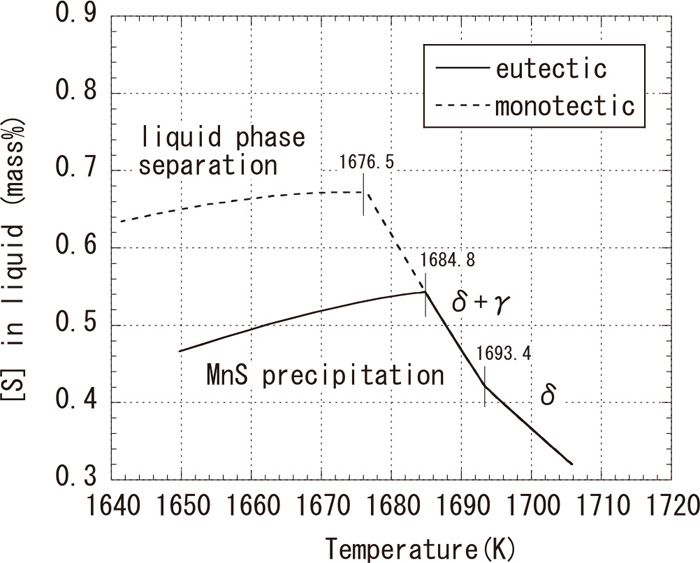
Microsegregation of [S] calculated by Scheil solidification model.
Microsegregation among the dendrite arms was analyzed by the Clyne-Kurz model20) using Eq. (1) to examine the influence of the cooling rate on the increasing of S content during solidification.
| (1) |
| δ | γ | ref. | ||
|---|---|---|---|---|
| liquidus temperature (K) | 1705.85 | 1698.81 | 13) | |
| liquidus slope (K/mass%) | C | −116 | −56 | 13) |
| Mn | −4.5 | −5.2 | ||
| S | −43 | −33 | ||
| Cr | 0.1 | −4.6 | ||
| Ni | −6.6 | −0.75 | ||
| N | −105 | −15 | ||
| partition coefficient | C | 0.12 | 0.26 | 13) |
| Mn | 0.77 | 0.78 | ||
| S | 0.061 | 0.018 | ||
| Cr | 1.0 | 0.82 | ||
| Ni | 0.76 | 1.0 | ||
| N | 0.25 | 0.65 | ||
| Gibbs-Thomson coefficient (mK) | 2.8×10−7 | 3.3×10−7 | 27) | |
| diffusion constant (m2/sec) | C | 1.43×10−7 | 28) | |
| Mn | 1.47×10−6 | |||
| S | 4.90×10−7 | |||
| Cr | 2.67×10−7 | |||
| Ni | 4.92×10−7 | |||
| N | 1.43×10−7 | |||
| activation energy (J/mol) | C | 4.69×104 | 28) | |
| Mn | 5.06×104 | |||
| S | 3.60×104 | |||
| Cr | 6.69×104 | |||
| Ni | 6.78×104 | |||
| N | 4.69×104 | |||
Liquidus temperature was estimated based on Howe equation,22) and ΔT was determined by convergence calculation. The calculation result (fs=0–0.4) for primary δ solidification is shown in Fig. 9. As described before, the dendrite arm spacing decreases as the cooling rate increases. The calculated segregation was reduced in the case of higher cooling rate due to the effect of back diffusion in the solid phase. The dimensionless diffusion time, α was larger in the case of 100 K/s. In addition, there was little difference over the range 10–100 K/s. It was difficult to explain the formation of metastable monotectic sulfides from the viewpoint of microsegregation, and subsequently analyzed the dendrite tip temperatures (undercooling) and the chemical composition at the dendrite tip.

Microsegregation of [S] calculated by Clyne-Kurz model.
Figure 10 shows the result of the dendrite tip temperature calculated by Eq. (2) based on the Kurz-Giovanola-Trivedi (KGT) model23,24,25,26) extended to a multicomponent system.
| (2) |
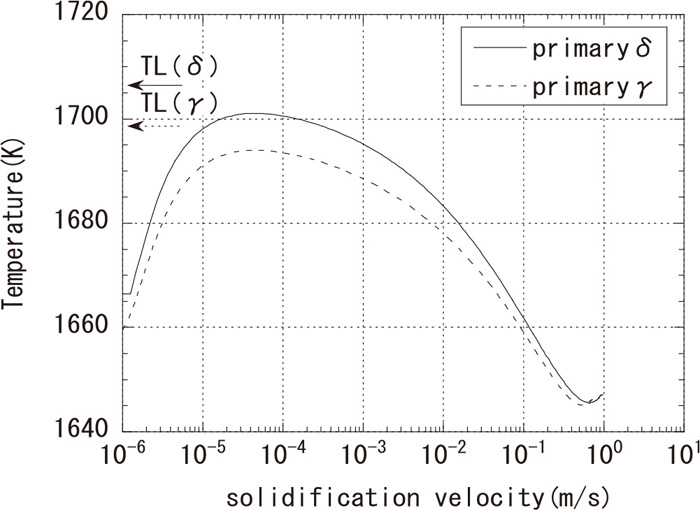
Calculated dendrite tip temperature (G=250 K/cm).
Components used for the calculation are the same as those shown in Fig. 7, and the equilibrium partition coefficient k and the liquidus slope m were obtained from the equilibrium calculation using PANDAT at the liquidus temperature and were assumed to be constant regardless of compositions and temperatures. Values from reference 27) were used for other physical properties and the data in reference 28) were used for the diffusion coefficients. Parameter values used in this calculation are shown in Table 3. Because the solidification cooling rate in Fig. 4 is relatively similar to that of a continuous casting billet, the solidification velocity was estimated as 0.2–5 mm/s and the temperature gradient was estimated to be 250 K/cm by the heat-transfer analysis of billet continuous casting. As a result, it is assumed that an undercooling of 7–18 K from the liquidus temperature occurs in the primary δ solidification. Undercooling of SUS303 is larger than that of commercial stainless steel. High concentration of alloy components in SUS303 and the low partition coefficient of S, as low as < 0.1 (0.061 for δ solidification and 0.018 for γ solidification) are considered to contribute to the second term on the right side of Eq. (2), which represents solute undercooling. Assuming a solidification morphology proposed by Inoue et al.29) in which the δ and γ phases grow independently, the difference in the dendrite tip temperatures between δ and γ becomes small with an increase in the solidification velocity, as shown in Fig. 10.
Figure 11 shows the relationship between the concentration of S in the liquid phase at the dendrite tip and the solidification velocity. It is believed that when the velocity is below 10 cm/s, undercooling and the concentration of S increase with an increase in the solidification velocity, and therefore, sulfides tend to precipitate. When the solidification velocity is large, S is presumed to be concentrated at the same level as shown in Fig. 8. The equilibrium calculation showed that monotectic sulfides can be formed when the chemical composition and temperature at the tip is the same as that for 5 mm/s solidification velocity of γ-dendrite.

Calculated [S] contents ahead of moving dendritic solid/liquid interface.
Monotectic sulfide formation has not been quantified yet. However, it is presumed that when the solidification velocity is large, the degree of undercooling and liquid-phase S concentration at the dendrite tip become high, and therefore, metastable monotectic sulfides tend to form.
3.5. Influence of Added ElementsAs described above, the addition of Al and Ca, which act as deoxidizers, has a large effect on the formation of nucleation sites. However, for the sample with Ca addition, all inclusions do not necessarily have the duplex structure shown in Fig. 5, and it is unclear that liquid-phase oxides were involved in the formation of all monotectic sulfides. In addition, the difference between samples No. 1 and Nos. 3, 4 cannot be explained clearly. Therefore, other factors should be considered.
Since Al-addition contributes to a decrease in the concentration of oxygen (O) by deoxidation, there is a possibility that O influences the thermodynamic properties of steel, as reported by Ishimi et al.8) It is known that monotectic sulfides are formed when the concentration of O increases in the case of carbon steel.30,31,32,33) Because O and Ca reduce the activity of S,34) the formation of eutectic sulfides may be affected by their addition. Figure 12 shows the distribution of chemical composition of monotectic sulfides in the sample with Ca (No. 3) analyzed by EPMA. Oxygen (O) was present in the sulfide and furthermore, some (Ca) and (Si) were also detected. Although it is not easy to imagine the state in melting, oxides may form earlier and dissolve in the sulfide. If the activity of the sulfides declined because of the dissolved O and Ca, formation of monotectic sulfides can be promoted.

Distribution of composition in MnS(L) sulfide (No. 3).
Morita et al.35) analyzed the multicomponent partition coefficients thermodynamically and obtained Eq. (3):
| (3) |
The following results were obtained by investigation of the sulfide morphology in SUS303 and examination of the formation mechanism of metastable monotectic sulfides:
(1) While metastable monotectic sulfides were formed in the surface layer, in which the solidification cooling rate and solidification velocity was large, stable eutectic sulfides were formed inside the ingot.
(2) Addition of Ca promoted formation of monotectic sulfides and addition of Al promoted formation of eutectic sulfides.
(3) Nucleation plays a role in the formation of monotectic sulfides from the viewpoint of the interfacial energy and formation of liquid-phase oxides is considered important.
(4) Not only the solutal pile-up at the dendrite tip and undercooling but also the decrease in the activity of S because of O and Ca addition can affect the selection between monotectic and eutectic sulfides.