2017 Volume 57 Issue 10 Pages 1698-1702
2017 Volume 57 Issue 10 Pages 1698-1702
In the steel refining process, dissolution of quick-lime would accompany the formation of 2CaO·SiO2 on its surface. For proper understanding of heat supply to the lime phase from the molten slag through this 2CaO·SiO2 layer, thermal conductivity of this phase should be well known. The present study investigates the thermal conductivity of 2CaO·SiO2 bearing solid solution by hot-wire and hot-strip methods. Thermal conductivity of the solid solution was obtained to have the value from 0.28 to 1.18 W/m·K at temperatures from 298 to 1623 K and found much smaller than that of CaO. Its temperature dependence was positive as is often characterized for complex oxide system. Addition of FeO as solid solution component to 2CaO·SiO2 phase raised thermal conductivity and effect of change in concentration of P2O5 was also elucidated to have a minimum peak of thermal conductivity appearing with this change. On the basis of the thermal conductivity of 2CaO·SiO2 obtained and that of CaO, thermal behavior of practical process has been evaluated. The complete thermal decomposition time of CaCO3 contained by 4 mass% in the sphere of quick lime having the radius of 1 cm is estimated to be about 30 s. The appropriate control of residual ratio of CaCO3 and size of quick-lime would be necessary for the promotion of lime dissolution into the steelmaking slag.
Hot metal pre-treatment process has been recently employed and carried out prior to the decarburization in the converter. Since this process is conducted at relatively low temperature, say c.a. 1350°C, retardation of lime dissolution into the slag has become a significant problem, which dissolution is essential for rapid and effective dephosphorization. Dissolution of lime into molten slag would proceed by two step reactions, namely, the formation of 2CaO·SiO2 on the surface or in the vicinity of the lime and dissolution of 2CaO·SiO2 into molten slag, where the rate-controlling step is regarded as mass transfer of 2CaO·SiO2 in the slag phase.1,2,3) To promote the dissolution of lime, elevation of temperature is a key factor because the solubility of 2CaO·SiO2 would greatly depend on the temperature and increase with its elevation. For proper understanding of thermal condition regarding lime dissolution, thermal conductivity of 2CaO·SiO2 is the most essential information. With this information, heat conduction from molten slag to solid lime would be appropriately understood. Nowadays, as the novel method for promotion of the lime dissolution, much attention has been paid and favorable result has been reported on the utilization of the gaseous phase generated from lime matrix.4) Carbon dioxide and steam gas generated by the thermal decomposition of CaCO3, that is remained limestone after calcination, and Ca(OH)2 which was formed by absorption of moisture in the air, can crush the 2CaO·SiO2 layer on the surface of the lime. These thermal decomposition reactions are endothermic; thus, supply of heat from molten slag to lime is essential and thermal conductivity is necessary to postulate this thermal behavior. Although thermal conductivities of CaO,5) FeO6) and CaO–Al2O3–SiO27) have been reported as important thermophysical properties, that for 2CaO·SiO2 has not been clarified yet. Hence, the present study aims to investigate the thermal conductivity of 2CaO·SiO2 to well describe the thermal behavior regarding the lime dissolution especially focused on the surface layer phase formed. In actuality, 2CaO·SiO2 forms accompanying the reaction of dephosphorization and/or oxidation of iron to become complex oxide with phosphorus oxide and/or iron oxide; thereby, pseudo binary solid solutions of 2CaO·SiO2-3CaO·P2O5 system and 2CaO·SiO2-2FeO·SiO2 system have been taken up for this measurement. From the thermal conductivity obtained, duration time of gas generation by thermal decomposition of CaCO3 has been estimated assuming particular thermal conditions and the rate-determining step of this thermal phenomena to indicate the controlling factor of the practical lime dissolution process.
The sample was made by sintering the compressed pellet which were synthesized by mixing CaO obtained by calcination of CaCO3 powder (purity of 99.5%), SiO2 powder (purity of 99.9%), 3CaO·P2O5 powder (purity of 99%), and FeO obtained by pre-melting of the powder mixture of iron and Fe2O3 (purity of 95%) in an iron crucible in Ar atmosphere for 1 h at 1673 K. Before this synthetization, preparation of 2CaO·SiO2 by sintering the compressed powder mixture of CaO and SiO2 was made in advance, which was submitted to aforementioned final synthetization. Each compression was made on the mixed powder to be in the shape of pellet form having the diameter of 24 mm and the height of 4 mm under the pressure of 3.53×103 N. Sintering was carried out in an Al2O3 crucible in an Ar atmosphere for 24 h at 1673 K. Table 1 shows the composition of the solid solution prepared by the foregoing manner to be used in the thermal conductivity measurement. This table is made on “mass%” basis and abbreviated to “%” hereafter. Indentification of the phase in the sintered sample was made by XRD analysis to confirm the formation of intended solid solution compound for 72%(2CaO·SiO2)-28%(3CaO·P2O5) solution as shown in Fig. 2. By sandwiching a platinum wire or strip with these pellet samples and putting the current in this platinum heater, the thermal conductivity of the sample was determined from the temperature elevation of the heater on the base of subsequent relation:
| (1) |
| (mass%) | ||
|---|---|---|
| 2CaO·SiO2 | 3CaO·P2O5 | 2FeO·SiO2 |
| 87 | 13 | 0 |
| 72 | 28 | 0 |
| 40 | 60 | 0 |
| 73.3 | 22.1 | 4.6 |
This method is called hot wire/strip method,8) which can determine the thermal conductivity on the bases of the tendency of heat diffusion from the heating material, namely, hot wire or strip, obtained by monitoring the temperature elevation. The schematic diagram of the experimental set-up is shown in Fig. 1.

Experimental set-up for the determination of thermal conductivity (Hot wire/strip method).
Figure 2 shows the XRD pattern analyzed for the sintered sample having the composition of 72%(2CaO·SiO2)- 28%(3CaO·P2O5). Although this 2CaO·SiO2 bearing solution significantly contains the component of 3CaO·P2O5, peak pattern shows very good agreement with the reported PDF data of 2CaO·SiO2; thus, the formation of 2CaO·SiO2 phase was successfully confirmed as was expected. Figure 3 shows the thermal conductivity obtained for the solid solution having the composition of 72%(2CaO·SiO2)-28%(3CaO·P2O5), together with that for CaO having different porosity. Values of about 0.65 W/(m·K) were obtained for this solid solution, which is less than half of the value obtained by Susa et al.7) for porous CaO having almost the same porosity of 0.36 as the present sample. Dense CaO5) has much higher thermal conductivity than porous CaO; thus, the thermal conductivity greatly decreases with an increase in porosity and complex oxide formation with SiO2 and P2O5 such as the present case.

XRD pattern for the sintered sample having the composition of 72%(2CaO·SiO2)-28%(3CaO·P2O5).
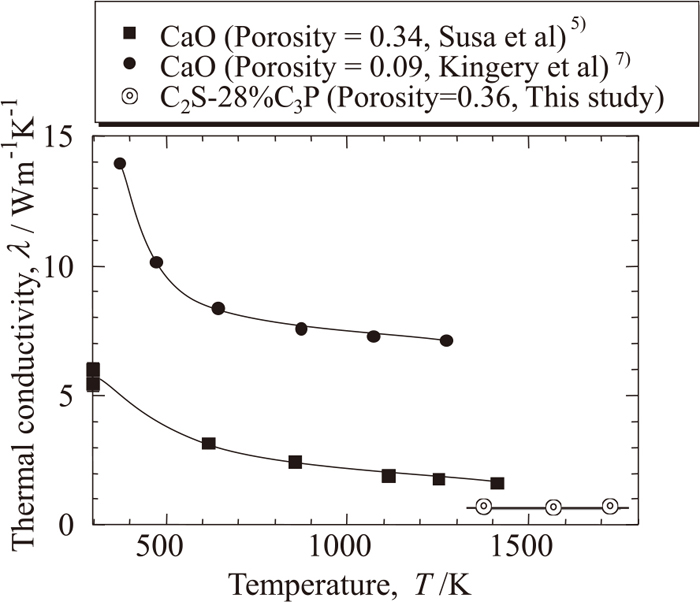
Thermal conductivity of CaO and the solid solution of 72%(2CaO·SiO2)-28%(3CaO·P2O5) as function of temperature.
For the practical refining process, iron oxide would be absorbed into 2CaO·SiO2 bearing solid solution. Figure 4 shows the temperature dependence of the thermal conductivities of solid solutions having the composition of 72%(2CaO·SiO2)-28%(3CaO·P2O5) and 73.3%(2CaO·SiO2)-22.1%(3CaO·P2O5)-4.6%(2FeO·SiO2), respectively. The thermal conductivity of the solution containing FeO is much higher than that without FeO, indicating that the practically formed 2CaO·SiO2 solid solution with FeO in refining process would be much more penetrable to heat than ideal one. The effect of FeO addition to the slag on the thermal conductivity was reported to be positive for the CaO–SiO2–FeO–Al2O3 system;9) thereby, the present results are considered reasonable. Thermal conductivity has a close relation with electrical conductivity especially for good conductors like metal since heat and electric current are carried mainly by free electron.10) For the case of slag, the same tendency may hold for the CaO–FeO system11) where the electric conductivity increases with an increase in FeO concentration, although free electron is not the main carrier of the heat and current. This would be supported by the fact that the electric conductivity of FeO is much higher than oxide of typical elements such as Al2O3.11) In case of such material having rather high electric conductivity as the present sample, leakage of the current from the heater, namely platinum wire or strip, should be well considered. Assuming that electric conductivity of the present solid solution of 73.3%(2CaO·SiO2)-22.1%(3CaO·P2O5)-4.6%(2FeO·SiO2) is 1 S·cm−1 on the basis of the data for CaO–SiO2 and FeOx–SiO2 system,11) the electric resistance of possible current penetration zone having the dimension of 5 mm×20 mm area (vertical to current direction) × 10 mm length (current direction) can be calculated to be c.a. 1 Ω, which is large enough compared with the resistance of the heater itself of 0.1 Ω. Therefore, the leakage of the current during the measurement can be judged not significant and the increase in thermal conductivity is attributed to solid solution of FeO. Figure 4 also show the positive temperature dependence for both systems. It is a characteristic trend for this complex oxide solid solution. Some of binary oxide systems such as K2O–SiO2 have positive tendency like the present results, but others such as MgO–SiO2 do the other way round.11) On the other hand, single phase material such as CaO5) and Fe2O36) shows the negative temperature dependence. At high temperatures of steelmaking process, the 2CaO·SiO2 bearing solid solution will have higher thermal conductivity, which promotes the heat conduction even more.

Thermal conductivity of 2CaO·SiO2 bearing solid solution with and without FeO as function of temperature.
Considering the dephosphorization process, P2O5 can be removed from the molten slag into the 2CaO·SiO2 bearing solid solution and its concentration would be changed and higher. Dependence of thermal conductivity of 2CaO·SiO2 solid solution without FeO on the concentration of 3CaO·P2O5 is shown in Fig. 5. The thermal conductivity shows a minimum peak along with the concentration of 3CaO·P2O5. It is also characteristic behavior but not unreasonable as is often the case with the complex oxide system consisting of basic oxide and acid oxide. For examples, CaO–P2O5, MgO–P2O5 and BaO–P2O5 system show a minimum peak for thermal conductivity at around 50 mol% of P2O5 composition.12) For the present case, it is not distinguishable which solid solution is more basic, 2CaO·SiO2 or 3CaO·P2O5, because these two components have been already combined and formed as the complex oxides of basic one and acid one; however, basicity, taken as the parameter, changes along with the concentration of 3CaO·P2O5, which change would result in the appearance of the minimum peak in thermal conductivity. Change in basicity with the composition of present solid solution may be proved by the fact that phosphorus distribution ration between molten CaO–SiO2–P2O5–FeO slag saturated with 2CaO·SiO2-3CaO·P2O5 solid solution and carbon saturated molten iron sharply decreases with an increase in 3CaO·P2O5 concentration of the solid solution.13)

Dependence of thermal conductivity 2CaO·SiO2-3CaO·P2O5 solid solution on the 3CaO·P2O5 concentration.
On the basis of thermal conductivities presently obtained, heat transfer and supply in the practical process can be discussed from the kinetic point of view. As mentioned in the foregoing chapter of the introduction, residual CaCO3 having survived the calcination process and remained in the quick-lime can be thermally decomposed to generate CO2 gas on heating, which effectively breaks the covering layer of 2CaO·SiO2 on the surface of lime. However, this thermal decomposition is the endothermic reaction, namely, heat absorbing reaction; thus, sufficient supply of heat from the molten slag to solid lime should be maintained. Considering the low thermal conductivity of 2CaO·SiO2 in comparison with CaO, and that 2CaO·SiO2 layer would be the heat resisting phase, the necessary time for the complete thermal decomposition has been estimated as follows. Suppose that there is a lime sphere having the radius of 1 cm (R) with the porosity of 0.36 (1-y) and the residual ratio of CaCO3 of 4 mass% (x) in molten slag, which sphere is being thermally decomposed and always covered with 2CaO·SiO2 layer as the heat resistant phase, where the rate-controlling step is assumed the heat transfer in the 2CaO·SiO2 layer. Inflow heat into the lime sphere is supposed to be completely consumed for the thermal decomposition of CaCO3 and no volumetric change is considered to occur through this reaction. The schematic image of this phenomenon is described in Fig. 6(a). On the basis of these condition, the complete decomposition time has been estimated as a function of the thickness of 2CaO·SiO2 layer (ΔR) by using the following physical properties of the ideal density of CaO, 3.32 g/cm(ρ),5) thermal conductivity of 2CaO·SiO2 of 0.65 W/m·K(λ), formula weight of CaCO3 of 0.1 kg/mol(m), and the standard Gibbs energy of decomposition of CaCO3 as follows:
| (2) |
| (3) 5) |
| (4) |
| (5) |

Schematic diagram of heat absorption from molten slag into lime sphere with the two cases of rate-controlling step; (a) heat transfer in the 2CaO·SiO2 boundary layer, (b) heat diffusion in the formed CaO layer.
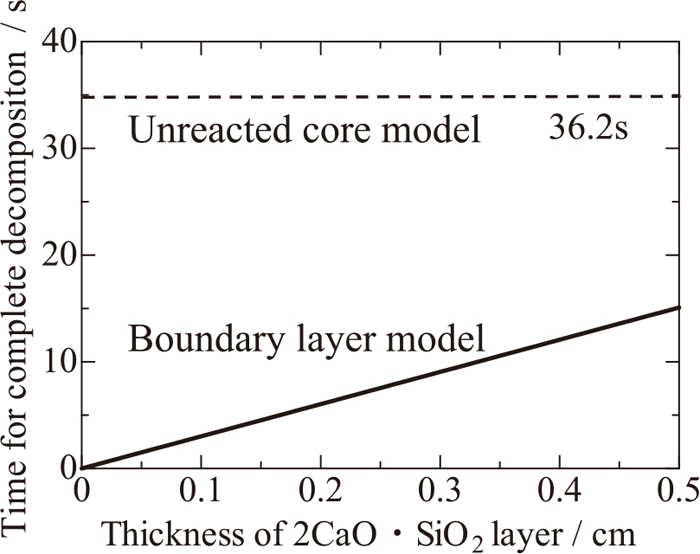
Duration time for gas generation until complete decomposition of spherical quick-lime containing CaCO3.
Thermal conductivity of 2CaO·SiO2 bearing solid solution was measured by hot-wire and hot-strip methods. The findings are summarized with the following conclusions.
(1) Thermal conductivity of 2CaO·SiO2 bearing solid solution is much smaller than that of CaO and has positive dependence on temperature.
(2) FeO solution to 2CaO·SiO2 phase raises thermal conductivity.
(3) Thermal conductivity of the phase changes with an increase in P2O5 concentration and shows a minimum peak.
(4) The complete thermal decomposition time is estimated to be about 30 s for the lime sphere having the radius of 1 cm and residual CaCO3 concentration of 4 mass% on the basis of the thermal conductivities of 2CaO·SiO2 obtained and CaO. The appropriate control of residual ratio of CaCO3 and size of quick-lime sphere would be helpful to the promotion of lime dissolution into the steelmaking slag.
The authors are greatly thankful to Prof. Masahiro Susa for his helpful advices and fruitful discussion.