2018 Volume 58 Issue 5 Pages 886-891
2018 Volume 58 Issue 5 Pages 886-891
Ferromanganese alloys are usually used for the manganese adjustment to meet the requirement of IF steel grade; and the formation and transformation of manganese-containing inclusions were closely relevant with the recovery yield of manganese in the melt and the morphologies of final deoxidation products. Characteristic transformation of manganese-containing inclusions during Al-killed process in ultra-low carbon interstitial-free steel were studied through the factory trial and hot crucible experiment. Factory trial showed that the manganese recovery yield fluctuated between 14% and 92% mainly due to the formation of manganese-containing inclusions. Hot crucible experiment revealed that three different groups of inclusions, including FeO·xMnO (FM), FeO–Al2O3 (FA) and Al2O3 (AO) inclusions, appeared after semi Al-killed; and five typical Al2O3 inclusions, including spherical Al2O3, polygonal Al2O3, cluster Al2O3, dendritic Al2O3 and aggregated Al2O3, appeared successively based on different formation mechanisms. Under homogenous nucleation condition, the spherical Al2O3 appeared firstly, and changed to be cluster Al2O3 by collision growth or transformed to be large dendrite Al2O3 by single-direction diffusion growth. Under heterogenous nucleation condition, the manganese-containing inclusions were transformed to Al2O3 inclusions in the following order: spherical FeO·xMnO → spherical FeO·xMnO with coarse surface → polygonal FeO·Al2O3 → polygonal Al2O3 → aggregated Al2O3.
Interstitial free (IF) steel is an attractive material in the automotive industry due to its good formability and ductility. In IF steel the interstitial atoms, such as carbon and nitrogen etc., are controlled in a very low level during the smelting process by a series of manufacturing techniques, especially RH decarburization and nitrogen removal.1,2,3,4,5) Meanwhile, the titanium or niobium is used to stabilize the residual carbon and nitrogen to make interstitial atoms free in the final matrix.6) As mentioned in references,7,8,9) most of defects on surfaces in cold-rolled sheet were relevant with the inclusions especially the macro-inclusions. To achieve good formability of IF steel,10,11) it is crucial to maintain ultra-low carbon, ultra-low nitrogen and the high steel cleanness.
For the Al-killed titanium stabilized interstitial-free steel, many reports focused on the evolution of Al2O3 and Al2O3–TiOx inclusions at different smelting stages,12,13,14,15,16) while less on the characteristic transformation of manganese-containing inclusions during Al-killed process. The manganese-containing inclusions were formed largely in the melt due to the addition of ferromanganese alloys. The formation and transformation of manganese-containing inclusions were closely relevant with the recovery yield of manganese in the melt and the morphologies of final de-oxidation products.17) Manganese is important for the ultra-low carbon steel, which affects the recrystallization behavior and annealing texture formation;18) and the reaction of manganese with complex oxides inclusions resulted in Mn-depleted zones, which promoted the nucleation of intragranular ferrite.19,20,21) Present study revealed the transformation mechanism of manganese-containing inclusions during Al-killed process by following the characteristic transformation of manganese-containing inclusions from FeO·xMnO to Al2O3 in the melt.
Factory trial was carried out in Qian’an steel works of China for a kind of IF steel grade (denoted as DC06 listed in Table 1) production through a route of 210-ton Basic Oxygen Furnace (BOF) → 210-ton Rheinsahl-Heraeus (RH) → 60-ton tundish → Continuous Casting (CC). High free oxygen and low carbon in the melt were maintained at BOF endpoint by rimmed tapping to guarantee a smooth decarburization in RH section. Mid-carbon ferromanganese is added during tapping from BOF to adjust the manganese content of the molten steel. Two locations were sampled, five minutes after ferromanganese addition and RH finished after Al-killed, to observe the characteristic transformation of manganese-containing inclusions during Al-killed process.
| Grade | C≤ | Si≤ | Mn | P≤ | S≤ | Als | Ti | N≤ |
|---|---|---|---|---|---|---|---|---|
| DC06 | 0.0015 | 0.02 | 0.13 | 0.009 | 0.010 | 0.03 | 0.07 | 0.002 |
Hot crucible experiments were carried out in a tubular resistance furnace to verify the transformation process from FeO·xMnO to Al2O3 by killing free oxygen step by step. The deoxidizers before final de-oxidation are insufficient for full killing of free oxygen, which make it possible to observe FeO·xMnO, Al2O3 and those ongoing transformation ones on the same specimen. The experimental process was described as follows: (1) 550 g master material, with chemical composition listed in Table 2, was melted in a 99.99% purity Al2O3 crucible under argon protective atmosphere; (2) The melt was maintained half an hour at 1600°C to make the chemical composition uniformly and then the Fe2O3 powder was added to adjust the free oxygen in the melt; (3) The free oxygen at different time were detected using oxygen probes; then samples were taken at different interval time after Al-killed using an 8 mm inner quartz tube (see Fig. 1). No extra ferromanganese alloy was provided during hot crucible experiment because the manganese content in raw material is closed to the target range.
| Element | C | Si | Mn | P≤ | S≤ | Al | Ni≤ | Cr≤ | Cu≤ |
|---|---|---|---|---|---|---|---|---|---|
| Range | 0.08 | 0.03 | 0.12 | 0.12 | 0.009 | 0.05 | 0.02 | 0.02 | 0.02 |
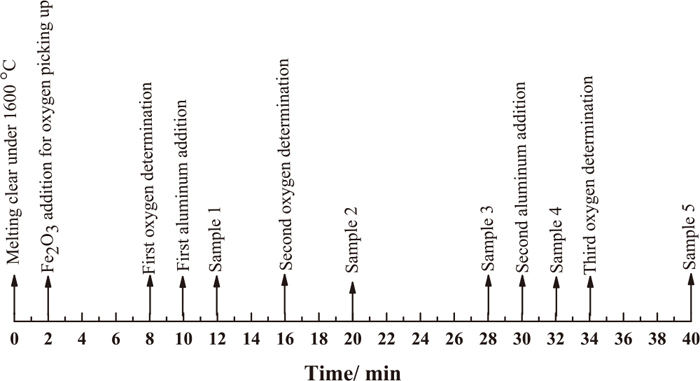
Sampling points of hot crucible experiment.
Bomb-shape sampler shown in Fig. 2 with inner diameter of 40 mm and height of 150 mm was used in factory trial. A two-meter-length rebar was welded on the side of the sampler during sampling from the ladle. The bomb-shape sampler was immersed into steel melt for 3 s by controlling the rebar through the slag layer quickly to prevent slag into the sampler. Two types of specimens including a block with size 15 mm*15 mm*10 mm for metallographic examination and two rods with size Φ5 mm*50 mm for inclusions extraction and total oxygen analysis were machined from the middle part of the bomb after cutting off the head and tail. Under the hot crucible experiment, rod-like specimens were prepared for the inclusions extraction and total oxygen analysis.

Bomb-shape sampler for sampling in ladle during industrial trial. 1. Slag cap which fixed using Ø 1 mm iron wire; 2. Bomb-shape sampler; 3. Rebar rod for sampling into the ladle. (Online version in color.)
Three-dimensional morphologies and compositions of inclusions are obtained by scanning electron microscope (SEM, ZEISS ULTRA55) and energy dispersive spectroscopy (EDS, OXFORD INSTRUMENTS INCA X-MAX50) after extraction with organic electrolyte solution22) and inclusions’ original morphology analysis treatment (IOA).23)
Mid-carbon ferromanganese, micro-carbon ferromanganese or electrolytic manganese are usually used for the adjustment of manganese levels in the melt at different stages of IF steel smelting.24) Mid-carbon ferromanganese, due to its high carbon content, is often used at BOF tapping to avoid carbon picking up in the melt; while with lower content of carbon, micro-carbon ferromanganese can be used in RH decarburization process and electrolytic manganese is mainly used after final de-oxidation. Although higher recovery yield can be achieved by using micro-carbon ferromanganese and electrolytic manganese for alloying during RH process, the high cost and large heat-loss restricted the usage amount. Mid-carbon ferro-manganese can be used to balance the cost of manganese alloying. The main problem for the alloying of ferro-manganese is to maintain stable and high manganese recovery yield without negative effects on carbon picking up.
Based on the tracking and analysis of 151 heats consecutive production data of DC06 as shown in Fig. 3, the manganese recovery yield of mid-carbon ferro-manganese fluctuated from 14% to 92% when it is used at BOF tapping, and the element manganese suffered serious losses during alloying process, which was relevant with the free oxygen in the melt, initial manganese content in the melt and addition amount of the ferro-manganese as shown in Fig. 4. The manganese recovery yield maintained from 60% to 80% by keeping the ratio of target manganese to initial manganese content ranging from 2.0 to 2.5 and free oxygen in the melt between 600×10−6−800×10−6. Low free oxygen is better for the manganese utilization, but is negative on RH decarburization in next sequence.
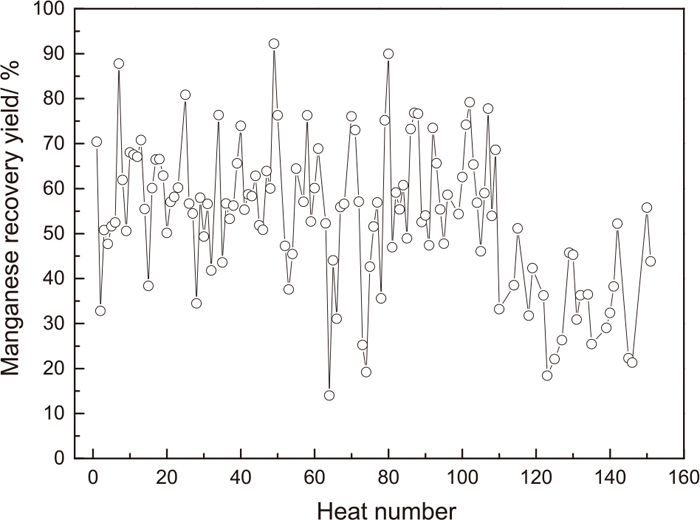
Fluctuation of manganese recovery yield after ferro-manganese alloyed at BOF tapping process.

Relationship between manganese recovery yield and ratio of manganese content before and after alloyed.
Figure 5 showed the morphologies of inclusions existing at 5 min after mid-carbon ferromanganese alloying. Most inclusions were spherical or ellipse FeO·xMnO with size below 5 μm. Here, the letter x represents the weight fraction of MnO to MnO and FeO in the inclusions with values ranging from 0.1 to 0.5. Some duel-phase FeO·xMnO inclusions with different values of x in inner core and outer shell in Fig. 5 indicated the formation of manganese-containing oxides at present condition due to the reaction of manganese and free oxygen in the melt.

Characterization of FeO·xMnO inclusions in molten steel at 5 min after ferro-manganese addition by methods a) metallographic observation and b) inclusions’ original morphology analysis (IOA) observation.23)
However, it is difficult to find the FeO·xMnO inclusions after Al-killed; almost all the FeO·xMnO inclusions changed to be different types of Al2O3, including spherical Al2O3 (Fig. 6(a)), polygonal Al2O3, (Fig. 6(b)), dendrite Al2O3 (Fig. 6(c)), and cluster Al2O3 (Fig. 6(d)) etc. The FeO·xMnO inclusions were reduced by soluble aluminum after Al-killed, which resulted in an average increment of 125 ppmMn in the melt. While, the manganese inside the inclusions were lost along with the floatation of inclusions, causing the fluctuation of manganese content in the melt.

Different types of Al2O3 inclusions including a) spherical Al2O3, b) polygonal Al2O3, c) dendrite Al2O3 and d) cluster Al2O3 after Al-killed.
Since large number of manganese-containing inclusions disappeared and transformed to be Al2O3 after Al-killed due to the rapid transformation condition as results showed in section 3.1, we designed hot crucible experiment to reveal the transformation process between FeO·xMnO and Al2O3 by killing the free oxygen in the melt step by step. The deoxidizers in previous steps were insufficient for fully killing of free oxygen; and it is possible to observe the FeO·xMnO and Al2O3 inclusions simultaneously under an insufficient Al-killed melt including those ongoing transformation ones in the same specimen.
Total oxygen in the melt is constituted with the free oxygen as dissolved oxygen and bond oxygen as oxides.25) The difference (ΔO) between total oxygen and free oxygen represented the amount of bond oxygen as oxides. As shown in Fig. 7, the free oxygen declines sharply after Al-killed, while the total oxygen decreased gradually, which indicated that the oxides in the melt presented a gradual decrement. Nearly 300 ppmO exist as bond oxygen at 16 min and it decreased to approximately 150 ppmO at 32 min. Thus, different types of inclusions including those of ongoing transformation ones were found in same specimen (see Fig. 8).

Change of the oxygen at different time of the hot crucible experiment.

Typical inclusions in one specimen of hot crucible experiment after partial Al-killed.
Three main groups inclusions including spherical FeO·xMnO (FM), spherical or polygonal FeO–Al2O3 (FA), and five typical morphologies of Al2O3 (AO) inclusions including spherical Al2O3, polygonal Al2O3, cluster Al2O3, dendritic Al2O3 and aggregated Al2O3 are summarized according to the chemical compositions and characteristic of the inclusions from different specimens. The FM inclusions mainly are spherical with size below 5 μm, a large portion of FM possessed rough surfaces and grooves on the surface as listed in Table 3 FM, and the characteristic of inclusions revealed the initial stage of transformation reactions between the FeO·xMnO and soluble aluminum; although element aluminum also appeared on the surfaces of FM inclusions, the content of Al2O3 is below 1 wt%. The FA inclusions mainly are spherical or polygonal with size below 5 μm with Al2O3 of 5–10 wt% and 20–30 wt% in spherical and polygonal FA inclusions respectively as listed in Table 3 FA. The AO inclusions mainly are Al2O3 with trace elements of Mn and Fe with sizes from ten microns to hundreds of microns as shown in Table 3 AO.
3.3. Transformation Mechanism of FeO·xMnO Inclusions after Al-killedDuring the Al-killed process, different types of inclusions were formed by reactions (1) and (2). The spherical FM inclusions are transformed to be FA or AO based on reaction (1) by heterogeneous nucleation. The free oxygen in the melt are transformed to be FA or AO based on reaction (2) by homogeneous nucleation. The FA or AO inclusions from reactions (1) and (2) possessed different characteristics. Spherical FA and AO or dendrite AO are easily formed by homogeneous nucleation at high concentration product of [Al] and [O]; polygonal FA and AO are mainly formed by heterogeneous nucleation based on reaction (1), as described in Fig. 9.
| (1) |
| (2) |

The characterization transformation of inclusions from FeO·xMnO to Al2O3 based on heterogeneous nucleation at different sampling points in Fig. 1 including a) sample 1, b) sample 2, c) and d) sample 3, e) sample 4, f) sample 5.
Normally, the initial nucleuses of Al2O3 when Al-killed were spherical and polygonal, then the nucleuses grow up to be a dendritic Al2O3 by diffusion growth or cluster Al2O3 by collision growth.26) Figure 10 showed some typical large-size Al2O3 inclusions in specimens after Al-killed including single-direction primary dendrite Al2O3 (Fig. 10(a)), multi-directional primary dendrite Al2O3 (Fig. 10(b)) based on diffusion growth, cluster Al2O3 (Fig. 10(c)) based on collision growth, and the mixture of cluster and dendrite Al2O3 (Fig. 10(d)) based on diffusion growth and collision growth both. Dendrite growth is directional, and the secondary dendrite is perpendicular to the primary dendrite of Al2O3. The growth of single-direction primary dendrite is due to the single-direction driving force of concentration gradient of elements Al and O; and the multi-direction driving force of concentration of elements Al and O results in multi-directional primary dendrite of Al2O3.

Typical large-size Al2O3 inclusions including a) single-direction primary dendrite Al2O3, b) multi-directional primary dendrite Al2O3 based on diffusions growth, c) cluster Al2O3 based on collision growth and d) mixture of dendrite and cluster Al2O3 based on diffusion growth and collision growth both.
Figure 11 presents the transformation mechanisms of different types of inclusions. Before Al-killed, the total oxygen in the melt existed in the forms of dissolved oxygen and FeO·xMnO oxides When aluminum was added into the melt, the dissolved oxygen and FeO·xMnO oxides reacted with soluble aluminum through the approaches of homogenous nucleation and heterogeneous nucleation respectively.
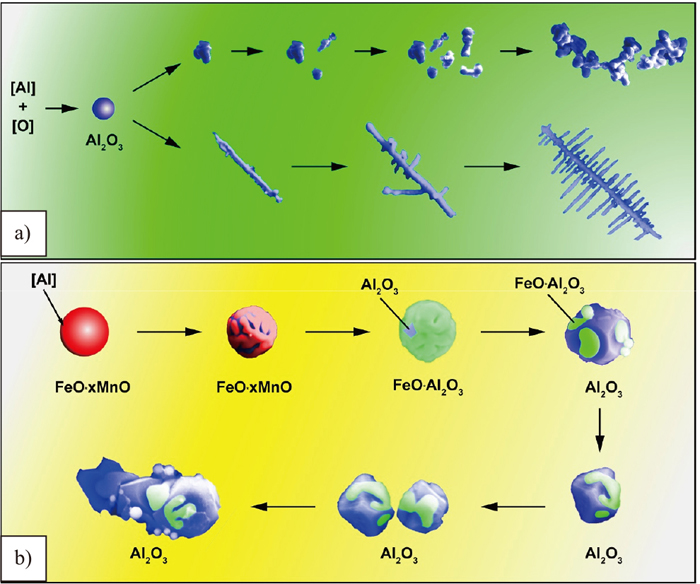
Transformation mechanisms of different types of inclusions by a) homogeneous nucleation and b) heterogeneous nucleation.
Under homogenous nucleation, the transformation mechanism of inclusions are described as follows: (1) A large number of spherical Al2O3 nucleus with size of 10 to 100 nm precipitated firstly due to the high supersaturation degree of [Al] and [O] after aluminum addition into the melt; (2) the spherical Al2O3 nucleus grow up to be a large spherical Al2O3 by homogenous diffusion or dendrite Al2O3 by directional diffusion until the supersaturation degree of [Al] and [O] disappeared; (3) the spherical Al2O3 inclusions collided with each other or with the dendrite Al2O3 to be a large cluster Al2O3 or mixture of dendrite and cluster Al2O3 due to the cavity bridge force.27)
Under heterogenous nucleation, the transformation mechanism of inclusions is described as follows: (1) The surfaces of manganese-containing inclusions grow coarse due to the partial reaction between soluble aluminum and oxide. (2) The spherical FeO·xMnO inclusions transformed to be polygonal FeO·Al2O3 by replacing MnO in the FeO·xMnO inclusions with Al2O3 through the reaction of aluminum with oxides. (3) the polygonal FeO·Al2O3 inclusions transformed to be polygonal Al2O3 by further reduction between inclusions and soluble aluminum. (4) The polygonal Al2O3 aggregate each other to be a large aggregated Al2O3.
The formation and transformation of FeO·xMnO inclusions were closely relevant with the recovery yield of manganese in the melt and the morphologies of final deoxidation products. The manganese recovery yield fluctuated between 14% and 92% after ferro-manganese alloyed in factory trial mainly due to the formation of manganese-containing inclusions. Hot crucible experiment was designed to reveal the transformation mechanism; and the characteristic transformation of manganese-containing inclusions after Al-killed transformed were described as follows: spherical FeO·xMnO → spherical FeO·xMnO with coarse surface → polygonal FeO·Al2O3 → polygonal Al2O3 → aggregated Al2O3.
This work was supported by the National Natural Science Foundation of China (No. 51404018) and the State Key Lab. of Advanced Metallurgy Foundation in China (No. 41617009). The authors wish to express their gratitude to the foundation for providing financial support.