2018 Volume 58 Issue 5 Pages 869-875
2018 Volume 58 Issue 5 Pages 869-875
Recently, interest in the art of dephosphorization in steelmaking has turned toward relatively lower basicity slag saturated with a [2CaO·SiO2-3CaO·P2O5] solid solution rather than CaO-saturated slag. In this work, laboratory experiments were carried out in order to investigate the change of the phosphorus-concentrated phase in low basicity slag. Phosphorus-containing slag was added onto 10 kg of hot metal, and its basicity was lowered through the desiliconization reaction of the hot metal at 1573 K.
EPMA observation of the slag after the experiment showed different mineral phases corresponding to the slag basicity (mass%CaO)/(mass%SiO2). When basicity was larger than 0.8, the phosphorus-concentrated phase (P-phase) was observed but was different from the solid solution phase in the initial slag. When basicity was lower than 0.8, the P-phase was not observed, and phosphorus was distributed through the slag at a low concentration. These results would reflect decomposition of the P-phase and formation of homogeneous liquid slag.
Based on a thermochemical consideration with the phase diagram of the [CaO–SiO2–FeO] system, the dissolution of the P-phase observed in the present experiment would be due to the slag composition approaching the SiO2 saturated region and a resulting decrease in CaO activity. From the viewpoint of the phase diagram of the [CaO–SiO2–P2O5] system, lowering the slag basicity to CaO·SiO2 saturation would come to coexistence of a higher P-phase such as 5CaO·SiO2·P2O5 or even 3CaO·P2O5, which would lead to an increase in the driving force for phosphorus transfer from slag to metal (so-called rephosphorization) at lower slag basicity.
At refining of phosphorus in hot metal, lime (CaO) is added as a dephosphorization (de-P) flux. The amount of added lime is controlled in terms of basicity (mass%CaO/mass%SiO2), which is given as a ratio against the amount of SiO2 generated by oxidation of silicon in hot metal. In general, de-P ability of high basicity slag saturated with CaO is high in thermochemical point of view. However, de-P operation with lower basicity slag is more beneficial with respect to resource reduction and slag utilization.
It is well known that 2CaO·SiO2-3CaO·P2O5 solid solution (hereafter denoted as 2CS-3CPss) is formed in slag containing CaO, SiO2 and P2O5 as a de-P product. 2CS-3CPss has wide solution range1) and exists as a solid within hot metal and molten steel temperature. With respect to the de-P slag containing 2CS-3CPss and liquidus phase (multiphase slag), many works as cited below have been done by Japanese research groups.
• Observation and analysis of reaction phase at the interface between solid CaO or 2CaO·SiO2 and molten slag2,3,4)
• Dissolution behavior of lime in P2O5 containing slag5)
• Dissolution behavior of solid 5CaO·SiO2·P2O5 into CaO–SiO2–FeOx slag6)
• Phase equilibria of CaO–SiO2–FeO–P2O5 slag under low oxygen potential7)
• Phase equilibria and activity of the component in CaO–SiO2–P2O5 ternary and CaO–SiO2–FexO–P2O5 quaternary system8,9,10,11,12)
• Evaluation of activity coefficient of P2O5 in 2CS-3CPss13)
• Phosphorus partition between 2CS-3CPss and molten slag14,15,16)
• Phosphorus distribution during solidification of phosphorus containing slag17)
With the lowering of slag basicity, 2CS-3CPss becomes thermochemically unstable, and phosphorus tends to be reduced from the solid solution in contact with molten iron or steel. Phosphorus transfer from slag to molten iron or steel is generally called re-phosphorization, which strongly affects an efficiency of de-P refining.18,19) However, there is little information on a critical composition for a stability of phosphorus-concentrated phase (hereafter denoted as P-phase) at lower basicity.
In practical de-P or decarbulization operation, phosphorus containing slag normally remains in reactor by sticking on the inside wall after previous heat. Such slag meets and is mixed with low basicity slag generated by predominant oxidation of Si in hot metal at the beginning of blowing. Change of P-phase in such low basicity slag should be understood in order to control de-P behavior in the early stage of blowing.
This paper aims to investigate the change of P-phase during lowering basicity of slag and the critical condition for stable coexistence of P-phase. Laboratory experiments were carried out to investigate the change of P-phase by making contact of phosphorus containing slag with low basicity slag formed during desiliconization of molten iron.
In order to investigate the change of mineral phase in slag during desiliconization of molten iron, desiliconization experiments were carried out using slag containing P-phase in a laboratory furnace. Schematic illustration of the experimental setup is shown in Fig. 1. The experimental condition is given in Table 1. At first, de-P experiments were carried out to obtain slag containing P-phase. 10 kg of molten iron containing Si and P was melted in a graphite crucible with a high frequency induction furnace. Fresh lime and iron oxide were added to molten iron followed by mechanical stirring with impeller. The de-P slag thus obtained was crushed and ground into powder under 250 μm. After that, another 10 kg of molten iron containing Si and P was melted in a graphite crucible with the high frequency induction furnace at 1573 K. 50 g of the slag powder and iron oxide were added to molten iron followed by weak stirring with impeller in order to avoid clumping of slag and to ensure reaction of Si in molten iron and iron oxide. Slag basicity is controlled by changing the initial composition of the slag, the initial content of Si in molten iron, and the amount of added iron oxide. In most experiments, the value of slag basicity became less than unity after the reaction time between 4–6 minutes. After the predetermined reaction time, the slag sample on molten iron was taken with iron rod.

Schematic illustration of laboratory experiment.
| Crucible | Graphite Inner diameter 140 mm × height 200 mm | |
| Stirring device | Impeller, 4 blades Width 50 mm × height 30 mm | |
| Stirring conditions | Immersion depth* 60 mm Rotation rate 600 rpm | Immersion depth* 60 mm Rotation rate 100 rpm |
| Metal | 10 kg (bath depth 100 mm) | |
| Temperature | 1573–1673 K | 1573±25 K |
| Initial composition | [Si]=0.05–0.15 mass% [P]=0.09–0.11 mass% | [Si]=0.05–0.15 mass% [P]=0.09–0.11 mass% |
| Flux composition | CaO (reagent grade) Iron oxide (reagent grade) | Dephosphorization slag Iron oxide (reagent grade) |
| Flux feed | Separately addition | Addition at beginning |
Elemental mapping and compositional analysis of P-phase of the initial and final slag were carried out with EPMA for evaluation of phosphorus distribution. Based on the analysis, change of P-phase was discussed in terms of phase diagram.
The bulk composition of the initial and final slag at desiliconization experiments is shown on [CaO–SiO2–FeO] ternary diagram in Fig. 2, based on sum of CaO, SiO2 and FeO being 100 mass%. In this figure isothermal section at 1573 K is shown by bold line. The slag basicity (mass%CaO/mass%SiO2) of initial slag ranges around 1.6–1.7, the content of FeO from 11–23 mass%, which correspond to 2CaO·SiO2 saturation.

Composition of slag before and after desiliconization. (projected on isothermal section of phase diagram of [CaO–SiO2–FeO] ternary system at 1573 K).
In Fig. 3, the bulk composition of the initial and final slag at desiliconization experiments is shown on a compositional triangle of [CaO–SiO2– P2O5] ternary system, based on sum of CaO, SiO2 and P2O5 being 100 mass%. In this figure one can see the macroscopic change of phosphorus content in the slag. The solid line connects the average composition of initial slag to SiO2 apex. This line corresponds to a compositional change in case of simple dilution of slag components by SiO2 mixing. The composition of slag after desiliconization experiments lies in lower P2O5 region than the solid line. Especially, the deviation of the slag composition from the solid line gets larger under the slag basicity less than 0.8. Therefore, it is suggested that the slag basicity below 0.8 would be an enhancing condition for decomposition of P2O5 in slag which leads to rephosphorization.

Composition of slag before and after desiliconization (projected on compositional triangle of [CaO–SiO2–P2O5] ternary system).
Typical EPMA images of the initial and final slag are shown in Figs. 4, 5, 6. The composition of P-phase in the final slag by quantitative analysis and the basicity of bulk slag are listed in Table 2.

Typical EPMA images of slag before desiliconization.

EPMA images of slag after desiliconization (No. 5 in Table 2) ((mass%CaO)/(mass%SiO2)=0.89).

EPMA images of slag after desiliconization (No. 8 in Table 2) ((mass%CaO)/(mass%SiO2)=0.63).
| Slag No. | Ca | Fe | Si | P | B* |
|---|---|---|---|---|---|
| 1 | 20.718 | 8.769 | 17.327 | 1.016 | 0.81 |
| 2 | 22.470 | 7.099 | 16.284 | 2.831 | 0.88 |
| 3 | 22.483 | 7.711 | 15.307 | 3.382 | 0.87 |
| 4 | 26.512 | 7.812 | 11.398 | 5.810 | 0.87 |
| 5 | 22.453 | 7.917 | 16.409 | 2.277 | 0.89 |
| 6 | 23.408 | 8.352 | 15.396 | 2.605 | 0.97 |
| 7 | 21.821 | 4.498 | 23.543 | 0.086 | 0.59 |
| 8 | 19.465 | 3.826 | 20.583 | 0.307 | 0.63 |
| 9 | 14.890 | 18.293 | 19.645 | 0.549 | 0.74 |
| 10 | 25.604 | 6.024 | 17.165 | 1.572 | 0.87 |
| 11 | 39.741 | 1.588 | 6.211 | 13.096 | 0.82 |
| 12 | 39.980 | 1.330 | 6.128 | 12.987 | 1.18 |
Figure 4 shows the elemental mapping image of the initial slag. P-phase is observed in the slag, the phosphorus content being 5.5 mass%. This phase has a similar composition to 2CS-3CPss, and their compositional ratio was about 73:27 in weight. The matrix phase surrounding P-phase would be liquid phase, the phosphorus content being as low as 1 mass% and the content of T. Fe + MnO as high as 20 mass%.
Figure 5 shows the elemental mapping images of the slag after the experiment, which corresponds to the slag No. 5 in Table 2. The bulk basicity of the slag is 0.89. One can observe the mineral phases with both high and low phosphorus content. The phosphorus content of the mineral phase with square shape is around 0.2 mass%, and this phase is identified as CaO·SiO2. The surrounding matrix phase contains about 2 mass% of phosphorus, which is higher than that of the bulk slag (1.4 mass%).
Figure 6 shows the elemental mapping images of the slag after the experiment, which corresponds to the slag No. 8 in Table 2, with the same initial slag shown in Fig. 4. The basicity of this bulk slag is 0.63. One can see that phosphorus is not concentrated in particular phase, which is almost uniformly distributed at the content not more than 0.2 mass%, as low as that of bulk slag. Therefore the P-phase in the initial slag would totally dissolve in this condition.
3.3. Change in Composition of P-phaseThe composition of P-phase observed in the initial and final slag with EPMA is plotted again on a compositional triangle of [CaO–SiO2–P2O5] ternary system in Fig. 7, based on the sum of CaO, SiO2 and P2O5 being 100 mass%. In the case that no P-phase is observed, the phosphorus content of the uniform liquid slag is employed. With respect to the P-phase in the final slag, two types of the phases are observed; one is relatively low basicity and lower P2O5 content than those in the initial slag, the other is relatively high basicity and higher P2O5 content than those in the initial slag. With respect to the former P-phase, the lower the basicity is, the lower the P2O5 content is.

Composition of phosphorus-concentrated phase before and after desiliconization experiment (projected on compositional triangle of [CaO–SiO2– P2O5] ternary system).
It is known that [CaO–SiO2–P2O5] ternary system is characterized by the existence of 2CS-3CPss.1) Most of the P-phase in the initial slag corresponds to 2CS-3CPss in spite of slight compositional deviation. The difference of the composition from stoichiometry would be due to minor elements in the phase such as Fe or Mn.
Consideration would be moved to the P-phase in the final slag which is lower slag basicity and lower P2O5 content than that in the initial slag. In the low basicity region of the ternary system, it is reported that solid CaO·SiO2, 2CS-3CPss and solid 3CaO·P2O5 would coexist at operation temperature of dephosphorization.20,21)
With respect to the P-phase in the final slag with P2O5 content ranging 5–10 mass%, the composition seems to be located near the tie line between CaO·SiO2 and 3CaO·P2O5 in spite of slight deviation. At EPMA observation of these slags, solid CaO·SiO2 almost free of phosphorus, and the matrix phase with relatively high phosphorus content and the basicity lower than unity are observed. Therefore, the composition of P-phase plotted in Fig. 7 regarding to these slags corresponds to the matrix phase which surrounds solid CaO·SiO2.
Based on the above discussion on these slags, it is considered that 2CS-3CPss in the initial slag would decompose into solid CaO·SiO2 and solid compound with high phosphorus content such as 3CaO·P2O5, as a result of lowering of the slag basicity during desiliconization reaction. At EPMA observation of the slag obtained after desiliconization experiments, 3CaO·P2O5 phase is not observed as a detectable size. This would suggest the possibility that this compound rapidly dissolves into the liquid slag. In the case of the slag in Fig. 5, part of 3CaO·P2O5 might react with molten iron and be reduced before detectable mineral phase grows, but some residue would remain in the slag. On the other hand, as shown in Fig. 6, most of the P-phase disappeared at lower slag basicity.
In Fig. 8, the isotherm at 1573 K of [CaO–SiO2–P2O5] ternary phase diagram is shown.20) Since P2O5 activity in 2CS-3CPss with high P2O5 content coexisting with CaO·SiO2 is not well known, the present results is discussed using previous works on the activity of P2O5 in phosphorus-containing compound in the system CaO–SiO2–P2O5. Hasegawa et al. reported the change in experimental and calculated P2O5 activity along tie line between 2CaO·SiO2 and 3CaO·P2O5 at 1573 K.21) For example, the P2O5 activity in 2CS-3CPss with mass%P2O5 = 9.0 which coexists with CaO and 3CaO·SiO2, is reported as −26.3 in logarithmic scale. The P2O5 activity of the solid solution increases with the content of 3CaO·P2O5, and its calculated value reaches up to −25.2 in logarithmic scale at the composition corresponding to 5CaO·P2O5·SiO2, which is the equimolar compound of 2CaO·SiO2 and 3CaO·P2O5. On the other hand, the activity of P2O5 for α-3CaO·P2O5, which is the end component of the solid solution, is obtained under coexistence with 4CaO·P2O5 as −23.7 in logarithmic scale at 1573 K.23)

Isothermal section of phase diagram of [CaO–SiO2–P2O5] ternary system at 1573 K.
In the above comparison, it is estimated that P2O5 activity in 3CaO·P2O5 is larger by two order than that of the P-phase of mass%P=10–17 (calculated in CaO–SiO2–P2O5 ternary composition) in the initial slag. Although the P-phase in the slag coexists with low basicity compound such as CaO·SiO2 in CaO–SiO2–P2O5 ternary system, once 3CaO·P2O5 is formed, P2O5 would be easily reduced in contact with carbon containing molten iron.
It is interesting to notice that the composition of the P-phase which has the highest basicity and the highest P2O5 content is similar to a stoichiometric compound 5CaO·P2O5·SiO2. The present authors deem that such phase reflect decomposition of 2CS-3CPss. The P2O5 activity in 5CaO·P2O5·SiO2 is referred under coexistence with CaO as −25.2 in logarithmic scale at 1573 K, which is lower than that of 3CaO·P2O5 as −23.7. Therefore, such decomposed phase similar to 5CaO·P2O5·SiO2 would react with molten iron relatively slow, and would remain as high P-containing phase in the slag during the experimental duration. In view of phase relationship, if the P-phase as 5CaO·P2O5·SiO2 is appeared by the decomposition, CaO·SiO2 could also be formed as a counter phase. However, CaO·SiO2 phase was not found in the slag as a detectable size. This would suggest that the P-phase as 5CaO·P2O5·SiO2 was observed at an intermediate state of phase variation under the increase of SiO2 during desiliconization, especially at the beginning of decomposition of 2CS-3CPss in the initial slag. In this case, the decomposition might precede the formation of detectable CaO·SiO2 phase.
In Fig. 9, the relationship between the bulk basicity of the slag and P2O5 content in P-phase is shown. With respect to the initial slag, there is no obvious relation between the slag basicity and the P2O5 content in P-phase. For the slag after the experiment, P-phase did not appear at the slag basicity below 0.8. At the slag basicity over 0.8, P-phase remained and P2O5 content in P-phase became higher steeply and some of them are even higher than that of initial slag. This results would be due to the process that 2CS-3CPss in the initial slag is decomposed into solid CaO·SiO2 and solid 5CaO·P2O5·SiO2 or solid 3CaO·P2O5 during desiliconization reaction. A mechanism of decomposition of 2CS-3CPss along with desiliconization, especially a transfer of 3CaO·P2O5 into surrounding matrix phase or solid CaO·SiO2, should be clarified as a future subject.
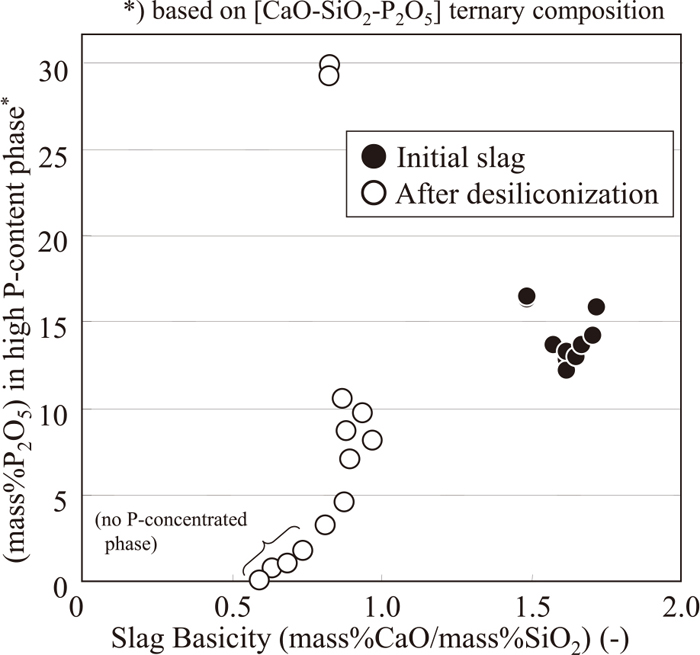
Relationship between (mass%P2O5) in phosphorus-concentrated phase in slag and slag basicity.
After the results at the desiliconization experiment adding phosphorus containing slag to molten iron, the phosphorus content in slag tends to become particularly lower beyond the slag basicity of 0.8. Following discussion for the influence of slag basicity would be possible here at this point.
In Fig. 10, the isothermal section at 1573 K of [CaO–SiO2–FeO] ternary phase diagram is shown. Following notation is employed; 3CaO·SiO2 = 3CS, 2CaO·SiO2 = 2CS, 3CaO·2SiO2 = 3C2S, CaO·SiO2 = CS.

Isothermal section of phase diagram of [CaO–SiO2–FeO] ternary system at 1573 K.
During the experiment, the composition of the initial slag moves from the region of 2CS saturation to the lower basicity region of CS saturation by desiliconization of molten iron. A region of SiO2 saturation appears for further lower basicity, in which region a driving force for re-phosphorization may be extremely larger. That is, under such low basicity condition, even dissolution of Ca-concentrated phase may be promoted.
In this diagram, boundary composition between the region of CS saturation and SiO2 saturation, as shown in Fig. 10, the lower the FeO content is, the higher the basicity of boundary composition is. The boundary of SiO2 saturation for slag on this diagram does not actually meet with that of complex slag at practical steelmaking operation, however, it would be important to consider a condition for avoiding dissolution of P-phase during lowering of slag basicity of commercial operation, based on such phase diagram.
In Fig. 11, the literature data for phosphorous partition ratio (Lp) of [CaO–SiO2–FeO] slag is shown.24) These Lp are taken along liquidus line of CS saturated region based on iso-Lp line on [CaO–SiO2–FeO] system reported in reference No. 24 and plotted against basicity. Although this figure shows only a comparison of data directly taken from the diagram in the literature, it is observed that Lp drastically decreases at the basicity below 0.9. This observation qualitatively agrees with the change in P-phase distribution in the slag around the basicity of 0.8 in aforementioned results.

Relationship between phosphorus distribution ratio and slag basicity (after Im et al.).
In Fig. 12, dependence of CaO activity on CaO content is shown for the region saturated with various [CaO–SiO2] compound, which appears along the line with FeO = 10 mass% in the isothermal section at 1573 K of [CaO–SiO2–FeO] ternary phase diagram. The constant activity values correspond to 3-phase coexistence of [CaO–SiO2–FeO] ternary system. In these region, since two [CaO–SiO2] compound and one liquid slag coexist, the degree of freedom is unity and therefore the activity is constant at a fixed temperature. The activity is calculated using the thermochemical data after Barin.25)

Dependence of CaO activity on CaO content in [CaO–SiO2] binary system at 1573 K.
One can find from the figure that the CaO activity decreases with the decrease of CaO content. The CaO activity steeply decreases with CaO content below 50 mass%, which corresponds to less CaO content of the region of 3C2S and CS saturation than the region of 2CS and 3C2S saturation. A decrease in CaO activity thermochemically results in increase in P2O5 activity and would promote re-phosphorization. As shown above, the particular change in phosphorous distribution in slag and Lp at slag basicity below 0.8 would qualitatively agree with such change in CaO activity.
In order to understand the change in phase relationship with compositional change of slag in the present system in more detail, a single line with constant P2O5 content is drawn in the isothermal section at 1573 K of [CaO–SiO2–P2O5] ternary phase diagram in Fig. 8, Let us consider the change in slag basicity from higher side to lower side along this line. Toward lower basicity from the region of CaO saturation to 3CaO·2SiO2 saturation, crossing the tie line of 2CaO·SiO2-3CaO·P2O5, 2CS-3CSss with the content of P2O5 below 7 mass% appears as a common coexisting phase, together with 3CaO·SiO2 or 3CaO·2SiO2 phase depending on bulk basicity. Further lowering of basicity to the region of CaO·SiO2 saturation results in coexistence of 2CS-3CPss with higher P2O5 content, In those narrow regions, CaO·SiO2 can coexist with 7CaO·P2O5·2SiO2, 5CaO·P2O5·SiO2 or even 3CaO·P2O5.
Considering a slag composition of mass% P2O5 = 3 (based on [CaO–SiO2–P2O5] ternary system) and basicity below 1.2 for example, mineral phase with higher P2O5 content can coexist as an equilibrium phase during lowering of basicity. At the present experiment, slag under the coexistence with 5CaO·P2O5·SiO2 phase could be observed. Further lowering of basicity, CaO·SiO2 and 3CaO·P2O5 would coexist. Although an information for phase diagram on the lower basicity region beyond tie line between CaO·SiO2 and 3CaO·P2O5 is not sufficient, some literature shows that pseudo-binary system of SiO2 and 3CaO·P2O5 is congruent system and both compound coexist below 1823 K.26) If such P2O5 rich phase would contact with molten iron, reduction of P2O5 in slag by carbon in molten iron might easily occur and lead to rapid re-phosphorization.
• Change of P-phase was examined by the experiment that phosphorus containing slag was added to 10 kg of molten iron at 1573 K and the basicity was lowered by desiliconization of molten iron.
• Observation of the phosphorus distribution and compositional analysis of P-phase in the initial and final slag was carried out using EPMA. The obtained results are as follows;
CaO/SiO2 > 0.8;
For the slag in which solid CaO·SiO2 is observed, the phosphorus content in matrix phase is relatively high. It is considered that 2CS-3CPss in the initial slag decomposed into solid CaO·SiO2 and solid compound with high phosphorus content such as 3CaO·P2O5, and the latter would dissolve in undetectable size into the slag. Some slag contained the compound with high phosphorus content up to 5CaO·P2O5·SiO2, which might reflect the intermediate state of decomposition of 2CS-3CPss in the initial slag.
CaO/SiO2 < 0.8;
No P-phase is observed and phosphorus is uniformly distributed throughout the slag. The P-phase in the initial slag would totally dissolved and homogeneous liquid slag would be formed.
• The reason for unstability of P-phase by lowering of slag basicity would be due to the lowering of CaO activity in [CaO–SiO2] system with the change in slag composition towards SiO2 saturation. This would lead an increase of driving force of re-phosphorization and considerable decrease in phosphorus distribution ratio, followed by the promotion of decomposition of P-phase in slag.
• Based on [CaO–SiO2–P2O5] ternary phase diagram, if the slag basicity lowers to CaO·SiO2 saturation, it is suggested that phosphorus containing compound with higher P2O5 content can coexist and leads to possible increment of driving force of re-phosphorization.