2018 Volume 58 Issue 7 Pages 1307-1315
2018 Volume 58 Issue 7 Pages 1307-1315
Distribution and morphology of MnS inclusions in as-cast ingots and as-forged bars of two Zr-bearing resulfurized non-quenched and tempered (NQT) steels have been performed. In the low Zr-bearing steel (0.001 wt%), MnS inclusions, which are teardrop-shaped or rod-like in two-dimensional (2D) morphology and dendritic or skeletal in three-dimensional (3D) morphology, are mainly distributed and segregated at the grain boundaries. While in the high Zr-bearing steel (0.0066 wt%), MnS inclusions are spherical or angular in both 2D and 3D observation and the distribution is more uniform than those in low zirconium steel. The calculated results by Thermo-Calc software show that the content of oxygen is not the direct factor that influences the morphology and distribution of MnS inclusions in medium-sulfur low-oxygen NQT steels. ZrO2 particles are ideal partcles for generation of spherical type I MnS inclusions owing to their strong nucleation capability and large amounts. However, the Zr and Al contents should be controlled cautiously to avoid generating large-sized agminated complex oxides, which are not easy to float and be removed owing to their high density. Otherwise, the ideal particles ZrO2 would decrease sharply in number and fail in offering sufficient heterogeneous nuclei for type I MnS inclusions. Besides, high proportion of complex MnS inclusions would decrease the supersaturation when pure MnS inclusions begin to precipitate, suppressing the generation of dendritic type II MnS inclusions.
Non-metallic inclusions in steels are generally harmful to their material performance, so extensive researches have been conducted to reduce their presence in steels.1,2,3,4) However, the utilization of inclusions for control of microstructures and enhancement of mechanical properties is becoming of considerable interest.5,6,7,8)
Manganese sulfide inclusions are typical inclusions detected in non-quenched and tempered (NQT) steels. Medium content of sulfur is usually added into steels for their beneficial effects on improving machinability,9,10) retarding grain growth11,12) and inducing intragranular ferrite.11,13,14) However, the large-sized elongated MnS inclusions, which exist in longitudinal section after deformation, are severely detrimental to the transverse mechanical properties of steels.15,16) Therefore, numerous studies focusing on the control of the morphology, size and distribution of MnS inclusions have been conducted over the years.17,18,19,20)
MnS inclusions have been classified into three types according to their morphologies in ingots, i.e., (1) randomly dispersed globular sulfides (type I), (2) rod-like or dendritic fine sulfides (type II), and (3) angular sulfides (type III).21) Type II sulfides are more flexible to be deformed and the most detrimental to the steel performance.22) While type I or III sulfides, which are more resistant to deformation and less harmful, are desired in medium-sulfur NQT steels.
It has been shown that the content of oxygen is the most significant factor that influences the morphology of MnS inclusions.23,24) Type I sulfides are generally seen in high-sulfur free-cutting steel with high oxygen content.25) While type II and III sulfides are usually generated in Al-killed steels with low oxygen content. However, the dispute still remains over which is directly responsible for the formation of type I MnS, the content of oxygen or the generated oxides. Yeo23,24) investigated the roles of deoxidants, deoxidation products and oxygen in the MnS morphology by addition of silicon, boron, aluminum and carbon (under vacuum). The results indicated that the oxygen was directly responsible for morphology change of MnS, and the actions of deoxidation and deoxidation products were actually indirect. Harttori et al.26) studied the influence of deoxidation on sulfide morphologies, and the results showed that in Ti-killed steel, the morphology of MnS inclusions would change from spherical to dendritic when oxygen contents decreased from 100 ppm to 50 ppm.
However, other researchers considered the deoxidation products were the key points. Oikawa et al.27) thought the Ti–Mn–O oxides were ideal nucleants for generation of type I MnS, even the oxygen contents in their specimens were less than 30 ppm. Isobe25) and Xia28) thought that the MnO oxides, which would be generated under condition of high oxygen content, could act as the nuclei when type I MnS inclusions begin to precipitate. In other words, they thought the deoxidation products were most crucial to the generation of type I MnS inclusions.
The resulfurized NQT steels, which possess excellent cutting performance, are usually used to manufacture the crankshafts or connecting rods of automobiles. But they have strict requirements on fatigue properties as well. The content of oxygen in the steels is usually less than 50 ppm.17,29) So, it could be impossible to obtain the type I MnS inclusions according to the theories proposed by Yeo or Harttori. However, if the deoxidation products are the key point of the formation of type I MnS, then, strong deoxidizer could be added into the steels for low oxygen content, only if the oxides were adequate and easily nucleated for type I MnS inclusions. Previous researches suggested that the steel with Zr addition would generate much larger number of oxides than most other deoxidizers under condition of similar oxygen content.30) Besides, the lattice parameters of ZrO2 particles and MnS inclusions are similar,31) which means MnS inclusions could be easily precipitated on the surface of ZrO2 particles.
So in this paper, different contents of Zr were added into two melts to figure out the key points that decide the MnS inclusions morphology and distribution in medium-sulfur low-oxygen NQT steels. The morphologies and distributions of MnS inclusions in as-cast ingots and as-forged bars were investigated. Besides, the content of oxygen and the effects of different component Zr-containing oxides on the MnS formation behavior were discussed.
Two 6 kg ingots of medium-sulfur NQT steels were manufactured in a MgO crucible by a vacuum induction melting furnace in laboratory. High purity raw materials were adopted to adjust the contents of C, Si, Mn, P, S and N. Pure iron, graphite block, electrolytic manganese and ferrovanadium were first melted, then alloyed by silicon metal, nitrogen-containing ferromanganese, aluminum block and zirconium sponge. Ferrous sulfides were last added for high yield. After homogenization of the composition, the molten steels were casted into a cast iron mould. Then, the ingots were stripped and cooled in air to the room temperature. The procedure of smelting is shown in Fig. 1.

Schematic diagram of the alloys addition sequence.
Each ingot was cut into two parts, as schematically shown in Fig. 2. The lower parts were prepared for investigation of MnS inclusions in as-cast ingots. The upper parts were homogenized at 1200°C for 2 hours and forged into bars with diameter of 40 mm, then cooled in air to the room temperature.

Schematic diagram of the sampling method.
The average compositions of the as-forged bars are listed in Table 1. The sample steel with lower Zr content is denoted as S1, the other sample steel with higher Zr content is denoted as S2. The contents of S in two sample steels are 0.043 wt% and 0.054 wt%, respectively. The content of O in S1 is larger than that in S2 and the values are 0.0062 wt% and 0.0032 wt%, respectively. In addition, the content of Al in S1 is higher than that in S2. It should be mentioned that the 0.0023 wt% Al contained in S2 is entirely from the impurities in alloys because aluminum block was not added in the S2. The contents of other alloying elements such as C, Si, Mn and V are similar in two sample steels.
| Steel | C | Si | Mn | P | S | V | Al | Zr | O | N |
|---|---|---|---|---|---|---|---|---|---|---|
| S1 | 0.37 | 0.60 | 1.45 | 0.0083 | 0.043 | 0.13 | 0.0052 | 0.001 | 0.0062 | 0.01 |
| S2 | 0.37 | 0.58 | 1.48 | 0.0075 | 0.054 | 0.12 | 0.0028 | 0.0066 | 0.0032 | 0.0098 |
The ingots and bars were subsequently cut into specimens with size of 12×12×7 mm3, followed by ground and polished, for preparing the inclusion inspection. In order to reveal the three-dimensional (3D) morphology of MnS inclusions, some specimens were electrolytically etched in an AA type electrolytic solution (10% acetylacetone, 1% tetramethyl-ammonium chloride and methanol).32) The electrolytic voltage was 30 V and the erosion time was about 60 s. A FEI Quanta MLA250 scanning electron microscope (SEM) was used to observe the two-dimensional (2D) and 3D morphologies of MnS and other inclusions. The chemical compositions of the inclusions were analyzed by energy dispersive X-ray spectrometer (EDS) connected to the SEM. Besides, an automatic analysis software, INCA was applied for statistical analysis of the number of MnS inclusions in longitudinal section of the bars. The minimum width of inclusions detectable was set as 0.5 μm and the total area detected in each sample was about 14 mm2.
Figure 3 has shown the different distributions and SEM morphologies of MnS inclusions in two as-cast ingots. In S1, the MnS inclusions are mainly distributed and segregated at the grain boundaries (Figs. 3(a) and 3(b)). The morphologies are teardrop-shaped or rod-like (Figs. 3(c) and 3(d)), which are categorized as type II MnS inclusions. In S2, the distribution of MnS inclusions is more uniform (Figs. 3(e) and 3(f)) and the morphologies are spherical or angular (Figs. 3(g) and 3(h)), which are of the types usually categorized as type I and type III. It should be noted that, a large proportion of MnS inclusions are actually precipitated on the surface of white nuclei, as shown in Fig. 3(i), which were identified as Zr-containing oxides by EDS analysis. Hence, these MnS inclusions are actually complex MnS inclusions.

2D SEM morphologies of MnS inclusions in ingots, a)–d) S1; e)–i) S2.
In order to investigate the 3D morphology of MnS inclusions, samples after being electrolytically etched were observed with SEM. The results are represented in Fig. 4. The MnS inclusions in S1 are mostly in dendrite shape and the lengths of primary dendrite are larger than 30 μm occasionally, which are quite different with the 2D SEM morphologies shown in Fig. 3. It can be inferred that the agminated teardrop-shaped or rod-like MnS inclusions shown in Fig. 3(c) or 3(d) would probably be the secondary dendrite of type II MnS inclusions shown in Fig. 4. A schematic diagram has been represented in Fig. 5 to describe the difference between 2D and 3D observation. However, the 3D morphologies of MnS inclusions in S2 are similar with the 2D morphologies. They are globular or angular in morphology, distribute homogeneously in the matrix. The sizes are about 2–4 μm in diameter.

3D SEM morphologies of MnS inclusions in ingots (after etched), a)–c) S1; d)–f) S2.

Schematic diagram of the difference between 2D and 3D observation.
Oxides + MnS inclusions, namely complex MnS inclusions, were detected with SEM-EDS in both as-cast ingots, as shown in Fig. 6. The results of EDS analysis show that the main chemical compositions of complex MnS inclusions in two ingots are both Zr-containing oxides + MnS inclusions. However, the chemical compositions of Zr-containing oxides are a little different and the oxides in S1 are more complex. The results of face scanning mapping indicate that the oxides in S1 consist of Al2O3 and ZrO2 oxides, and Al2O3 oxides are dominant. It is obvious that the Zr-containing oxides in S1 are the results of aggregation behavior of Al2O3 oxides, because ZrO2 particles are separated with each other. Besides, the complex MnS inclusions in S1 are spherical or regular in shape, which should be classified into type I or type III.

SEM morphologies and face scanning mapping of complex MnS inclusions in ingots, a)–b) S1; c)–d) S2.
In S2, the chemical compositions of Zr-containing oxides are relatively simple. Only one or two pure ZrO2 particles exist inside of the MnS inclusions. Occasionally, Al2O3 particles with size about 0.1–0.3 μm in diameter are also visible at the edge of ZrO2 particles, as shown in Fig. 6(d).
3.3. Distribution and Morphology of MnS Inclusions in As-forged BarsMnS inclusions possess high ductility and would be easily lengthened during forging or rolling process.17) Hence, the 2D morphologies of MnS inclusions in as-forged bars were investigated using SEM-EDS and the results are shown in Fig. 7. In S1, the MnS inclusions are strip and chain-like, which are common in commercial steels and have a severe adverse effect on transverse mechanical properties.15,16) The largest MnS inclusions are about 20 μm in length. In S2, the MnS inclusions are spindle in morphology and equally distributed. As seen in Figs. 7(e) and 7(f), there are plenty of MnS inclusions with wrapped white ZrO2 particles.
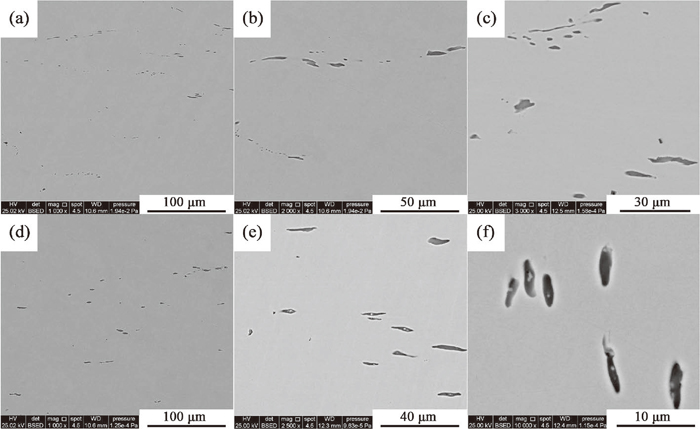
2D SEM morphologies of MnS inclusions as-forged in bars, a)–c) S1; d)–f) S2.
Figure 8 shows the 3D morphologies of MnS inclusions in as-forged bars. The MnS inclusions in S1 are strip and agminated. The lengths are more than 50 μm occasionally. Some dendritic structures are retained, as seen in Fig. 8(c), probably because the deformation direction was just perpendicular to the direction of primary dendrite. In contrast, the distribution of MnS inclusions in S2 is more uniform. The 3D morphologies of MnS inclusions in S2 are also similar with the 2D morphologies, i.e. strip or spindle in shape. Their lengths are about 5–10 μm.

3D SEM morphologies of MnS inclusions in as-forged bars (after etched), a)–c) S1; d)–f) S2.
Figure 9 shows the morphologies of complex MnS inclusions found in two as-forged bars. In S1, the complex MnS inclusions are spindle-shaped and large-sized. The chemical compositions of the internal oxides are similar with the oxides found in S1 ingot, which are comprised of ZrO2 and Al2O3 oxides. But the aggregation behaviors of oxides are more serious and obvious. In S2, the chemical compositions of the internal oxides are simpler, which are single pure ZrO2, or ZrO2 with tiny Al2O3 oxides.

SEM morphologies of complex MnS inclusions in as-forged bars, a)–c) S1; d)–f) S2.
Figure 10 has shown the number of pure MnS inclusions and complex MnS inclusions detected in two bars with INCA system. Because some MnS inclusions were precipitated in matrix after solidification,33) only MnS inclusions with size larger than 1 μm were counted. In S2, the complex MnS inclusions account for 48.5% of total MnS inclusions, which means nearly half of MnS inclusions in S2 were heterogeneous nucleation. It should be mentioned that some complex MnS inclusions could be regarded as pure MnS inclusions because the wrapped oxides might not be exposed during the specimen preparation, as schematically shown in Fig. 11. However, the proportion of complex MnS inclusions in S1 is only 3.2%. That indicates that almost all MnS inclusions in S1 were homogeneous nucleation.

Number density of pure MnS inclusions and complex MnS inclusions in the sample steels.

Schematic diagram of a pure MnS inclusion which is actually complex MnS inclusion.
It should be pointed out that the color of pure ZrO2 oxides is almost the same as the steel matrix and their sizes are quite small, causing trouble in finding those oxides in specimens merely polished. But the pure ZrO2 oxides exist indeed and the oxide detected in S2 specimen etched by 3 volume% nital solution is shown in Fig. 12. However, it is not convenient to count the number of these pure ZrO2 oxides and the results are imprecise as well. In order to estimate and compare the number density of oxides in two sample steels, 50 complex MnS inclusions in each sample were selected and the lengths of oxides inside of complex MnS inclusions were measured. It should be mentioned that all oxide precipitates inside of each complex inclusion are regarded as one complex oxide because they only offered one nucleation site for MnS inclusions. The average lengths of oxides in two samples are 4.16 μm and 0.82 μm, respectively, and the result is represented in Fig. 13. That means owing to aggregation behavior of oxides, which is shown in Figs. 6 and 9, the oxides in S1 are almost five times as big as these in S2. Hence, it can be inferred that the number of oxides in S2 is greatly larger than that in S1.

SEM micrograph and EDS analysis of pure ZrO2 oxides in S2.

Average length of oxides inside of complex MnS inclusions in two samples.
Dissolved oxygen content (DOC) has been considered to be the most important factor that influences the morphology of MnS inclusions in high-sulfur free-cutting steels.23,24,25) In order to investigate the change of DOC in two sample steels, the Thermo-Calc thermodynamic software was applied using the TCFE7 database. The calculated results between 1400°C–1600°C are shown in Fig. 14. The liquidus and solidus temperatures in two samples are basically the same and the values are 1490°C and 1420°C, respectively. MnS inclusions would be generated at the final solidification region and the temperature is about 1425°C. The total contents of oxygen in two sample steels are 0.0062 wt% and 0.0032 wt%, respectively. It can be seen that the DOCs in both steel samples decrease with the decrease of temperature and reach zero at about 1420°C. In addition, the DOC in S1 keeps higher than that in S2 from 1600°C to complete solidification. Moreover, the DOCs are quite low when MnS inclusions begin to precipitate since the contents of deoxidizers, such as Al, Zr, and Si are high in both sample steels. However, the morphologies and distributions of MnS inclusions are quite different as previously mentioned. So it can be concluded that the content of oxygen is not the direct factor that influences the morphology and distribution of MnS inclusions in medium-sulfur low-oxygen NQT steels.

Variations of oxygen content in sample steels.
As reported by Dahl34) and Oikawa,32) the type II MnS is considered to be formed through eutectic reaction. The Fe–MnS pseudo-binary phase diagram containing 1.4 wt% Mn and 0.38 wt% C calculated by FactSage software are shown in Fig. 15. With the cooling process (dashed in Fig. 15), the Fe-rich melt (L1) would precipitate δ–Fe first. In the meantime, the constituent of Fe-rich melt (L1) would variate along with the line xe. When the temperature reaches eutectic point (e), the eutectic reaction (L1 → Fe + MnS (s)) would happen and type II MnS inclusions are formed. Therefore, if there are no other particles existing in steels or steels are quite clean,35) only type II MnS inclusions would be generated. And they would be mainly distributed along the grain boundary because they are formed in the final solidification region.

Fe–MnS pseudo-binary phase diagram.
Generally, Al2O3 oxides are inclined to aggregate and easy to float and be removed.36) But the ZrO2 oxides in S1, which have high density,37,38) suppressed the floatation of the complex oxides. As a result, lots of large-sized complex oxides were retained in S1 and the O content is as high as 62 ppm, but the number of oxides is actually quite small. In a sense, the S1 is quite clean for MnS nucleation and the ratio of heterogeneous nucleation shown in Fig. 10 turns out to be only 3.2%. As a result, the MnS inclusions in S1 grow up with Fe in a eutectic way and are mainly distributed along the austenite grain boundaries.
In order to explain the generation of the spherical type I MnS inclusions, a metastable phase diagram was invoked by Oikawa.32) They proposed that the liquid MnS (L2) would be generated though monotectic reaction (L1 → Fe + MnS (L2)). Besides, they thought the liquid oxides, such as Ti–Mn–O system, were more effective to act as nucleation catalysts for MnS (L2) rather than MnS (s). However, oxides with high melting point, such as Al2O3 and TiN were the opposite. It is easy to understand the promotion effect of liquid oxides. The forming processes of MnS (L2) on the Ti–Mn–O27) and SiO2–MnO39) oxides have been described in detail by other researchers. But there was no more explanation for high melting oxides. Recently, a lot of oxides with high melting point, like ZrO2,31,40,41) Ce2O341) and Al2O3–MnO,42) were found to be effective as nucleation sites of MnS (L2). As seen Figs. 3 and 6, most MnS inclusions in S2 with ZrO2 inside are spherical, indicating the monotectic reaction has happened. Moreover, Fig. 16 shows more 2D morphologies of MnS inclusions observed in S1 ingot. Most MnS inclusions are rod-like, which are similar with the morphologies shown in Fig. 3. However, it is meaningful to find that one MnS inclusion in each figure is spherical in shape. The results of EDS analysis show that the internal white dots are ZrO2 oxides. There are not any oxides found inside of the other MnS inclusions. So, it can be concluded that it was the ZrO2 particles that changed the MnS morphology by promoting metastable monotectic reaction.

2D SEM morphologies of MnS inclusions in S1 ingot.
Therefore, it is reasonable to infer that the melting points of oxides are not the dominating reason for promoting eutectic or monotectic reaction. If the oxides possess high nucleation ability for MnS, metastable monotectic reaction would be preferred. Otherwise, eutectic reaction would occur and type II MnS would be generated. As for low melting point oxides, sulfur capacity of oxides could be adopted to assess the nucleation ability for MnS generation.33,35,43) As for high melting point oxides, low lattice misfit with MnS could be used to evaluate the nucleation capability. Ohta and Suito18) have calculated the lattice misfits of oxides, nitrides, carbides and sulfides with MnS, the values of ZrO2 and Ce2O3 particles are both less than 5%, indicating they are desired nucleation catalysts for MnS. However, the values of Al2O3 and TiN particles are both more than 10%, which are not considered as ideal nucleating agents of MnS generation. The calculation results are consistent with the researches of Oikawa.32)
The number of desired oxides is equally crucial to the monotectic reaction. If there are not enough oxides, it’s unlikely to obtain abundant spherical type I MnS. So, it can be explained that even the Ti–Mn–O oxides had been proved to be the perfect nuclei for MnS (L2) in Harttori’s study,26) when the oxygen in steels decreased from 100 ppm to 50 ppm, the morphologies of MnS have changed from spherical shape to dendrite shape. Similar results were also obtained after Al deoxidation and vacuum deoxidation.26) In low-oxygen steel, the most effective method to increase the oxides number is to refine the oxides size. In S2, the average size of ZrO2 particles is only 0.82 μm and the heterogeneous nucleation ratio of MnS inclusions is as high as 48.5%. As a result, the MnS inclusions are spherical and more equally distributed.
Therefore, Zr addition into molten steel as deoxidizer is recommended to control the morphology and distribution of MnS inclusions in low–oxygen NQT steels. However, it should be cautious to control the Zr and Al contents in molten steel. As can be seen in S1, it is hard for agminated Al2O3–ZrO2 complex oxides to float and be removed owing to their high density. Consequently, the oxides, some of which are more than 10 μm in length, remained in matrix, causing great detriment to the steel performance. More seriously, the ideal particles, i.e. ZrO2 oxides would decrease sharply in number and fail in offering sufficient heterogeneous nuclei for MnS inclusions.
4.3. Effect of Supersaturation on the Morphologies of MnSIt should be mentioned that about half number of sulfides in S2 are pure MnS inclusions, which are in spherical or angular shape rather than dendrite shape. This can be explained based on the crystal growth theory. Sunagawa44) investigated the relationship of supersaturation, growth rate and morphology of crystals. The results showed that with the increase of the supersaturation, the growth rate would increase and crystal morphology would change from regular to dendritic.
As we know, heterogeneous nucleation is more likely to occur than homogeneous nucleation due to the less nucleation energy needed, which means complex MnS inclusions would be generated earlier than the pure MnS inclusions. In S2, about half content of S element is in the form of complex MnS inclusions. In contrast, almost all S element in S1 is in the form of pure MnS inclusions. That indicates that when pure MnS inclusions begin to precipitate, the supersaturation of S element in the residual liquid steel of S2 would be much lower than that in S1. As a result, the growth rate of pure MnS inclusions in S1 is faster and eventually transform into the whole dendrite shape, while pure MnS inclusions in S2 are in angular shape due to their low growth rate. So, as reported before, with the increase of the S content,45) or the increase of the cooling rate,46,47) more type II dendrite MnS inclusions would be generated.
To sum up, MnS inclusions (L2) are first generated on the Zr-containing oxides, forming spherical type I MnS. Then, MnS inclusions (s) are precipitated through eutectic reaction in the residual liquid steel. In S2, the supersaturation of S element in the residual liquid steel is lower and the growth rate is slower, resulting in angular type III MnS. In S1, the supersaturation is higher and the growth rate is faster, generating a large number of dendrite type II MnS inclusions.
In this paper, different contents of Zr alloys were added into the melts to investigate the effect of Zr-containing oxides on the distribution and morphology of MnS inclusions in resulfurized NQT steels. The following conclusions are obtained.
(1) The content of oxygen is not the direct factor that influences the morphology of MnS inclusions in medium-sulfur low-oxygen NQT steels. The oxides with strong nucleation capability and large amounts are ideal particles for generation of spherical, evenly distributed type I MnS inclusions.
(2) Zr addition into molten steel as deoxidizer is recommended to control the morphology and distribution of MnS inclusions in low-oxygen NQT steels. But the contents of Zr and Al should be controlled cautiously to avoid generating large-sized agminated complex oxides. Otherwise, the ideal particles ZrO2 would decrease sharply in number and fail in offering sufficient heterogeneous nuclei for type I MnS inclusions.
(3) High proportion of complex MnS inclusions promoted by ZrO2 oxides would decrease the supersaturation when pure MnS inclusions begin to precipitate. Consequently, the dendritic type II MnS inclusions are suppressed and angular type III MnS inclusions are generated.
The authors are grateful for support from State Key Laboratory of Advanced Metallurgy (Project No. 41617023 and No. 41616015). The authors also appreciate the Xining Special Steel Co. Ltd. for the technical help.