2019 Volume 59 Issue 10 Pages 1786-1795
2019 Volume 59 Issue 10 Pages 1786-1795
Ore-based steelmaking generates various residues including dusts, sludges, scales and slags. Recycling of these residues within the process or via other applications is essential for sustainable production of steel. In blast furnace (BF) ironmaking, the gas-cleaning equipment generally recovers the particles in the off-gas as dust and sludge. Traditionally, the dry dust is recycled via the sinter or, in the case of pellet-based BF operation, via cold-bonded briquettes and injection. As the BF sludge mainly consists of iron and carbon, this residue is of interest to recycle together with the BF dust. However, depending on how the BF is operated, these two residues are more or less the major outlet of zinc from the furnace. Thus, to limit the recycled load of zinc, both materials cannot be recycled without dezincing the sludge prior to recycling. Dezincing and recycling of the low-zinc fraction of BF sludge via sinter have been reported whereas recycling via cold-bonded briquettes has not been performed. In the present study, cold-bonded briquettes containing the low-zinc fraction of dezinced BF sludge were charged as basket samples to the LKAB Experimental Blast Furnace (EBF). The excavated basket samples from the quenched EBF suggested that additions of up to 20 wt.% of upgraded BF sludge was feasible in terms of reducibility and strength. Based on these results, BF sludge were added to cold-bonded briquettes and charged in industrial-scale trials. The trials indicated that the annual generation of BF sludge, after dezincing, could be recycled to the BF.
Domestic environmental legislation,1) limited landfill areas and the cost of raw material and energy drive efforts towards increased recycling of in-plant residues from the ore-based steelmaking industry. These residues include dusts, sludges, scales and slags and recycling within the existing process or via other applications is essential for sustainable production of steel. However, the recycling has to be feasible from an economic, environmental and technical standpoint.
The BF is the dominating production unit used in ore-based ironmaking. The ferrous burden is charged at the top of the furnace as agglomerates in the form of sinter and/or pellets; in addition, lump ore may constitute part of the ferrous burden. Depending on the type of ferrous burden used and the on-site possibilities of agglomeration, different options for recycling of in-plant residues by top charging are possible. When operating on sinter, in-plant residues such as dusts, mill scales and micro-pelletized sludge can be incorporated in the sintering process.2) Also, fractions of crushed BOF slag are recycled via the sinter functioning as slag former.3) Steel plants without on-site sinter plants can use cold-bonded briquettes for recycling. These types of briquettes have successfully been used to recycle BF dust, screened fines of BF additives, filter dust, pellet fines, briquette fines, coarse and fine BOF sludge, fines of the magnetic part of the desulfurization slag (desulfurization scrap), steel scrap fines, mill scale and pickling sludge in industrial scale.4) Injection of e.g. BF dust via the tuyeres of the BF is also a possible route in addition to the recycling via top-charged agglomerates.4) Regardless of the recycling routes applied in the BF, the impurity levels of e.g. zinc limits the amounts of residues that can be recycled.
The BF dust is collected in the dust catcher or dry cyclone when treating the top-gas from the BF and this residue is usually recycled with no need for landfill. In addition to the coarse and dry dust, a wet finer residue is collected after the scrubber; namely, the BF sludge. The BF sludge is generally landfilled despite having a chemical composition dominated by iron and carbon. Three characteristics of the BF sludge distinguish why the BF dust is recycled whereas the sludge is not. These characteristics are the zinc content, the water content and the fine particle size distribution of the sludge. If both residues were to be recycled simultaneously, the zinc content is the limiting factor. Depending on how the BF is operated, the top-gas dust and sludge are more or less the main outlets of zinc from the BF. Therefore, if the dry dust is recycled to the BF, the sludge needs to be treated to remove zinc before recycling. This upgrading mitigates the accumulation of zinc in the BF. The negative effects of high loads of zinc in the BF include increased consumption of reducing agents, reduced life of carbon-based refractories and scaffold formation, which may ultimately lead to disturbances in the burden descent.5)
Upgrading of BF sludge and recycling of the low-zinc fraction via sinter6,7,8,9) and cold-bonded pellets9) have been realized in industrial-scale operation. When operating a BF on pellets and utilizing cold-bonded briquettes for recycling of in-plant residues, a logical recycling route for the low-zinc fraction of the BF sludge is via the existing infrastructure of briquetting. However, there are no reports on this recycling route. In the present paper, the feasibility of recycling the low-zinc fraction of upgraded BF sludge via cold-bonded briquettes was studied via basket samples charged into the LKAB EBF. In addition, non-treated BF sludge was charged via cold-bonded briquettes in industrial-scale BF trials to simulate the addition of upgraded BF sludge to the BF in full-scale operation.
Newly produced BF sludge from the integrated steel plant SSAB Oxelösund was upgraded, generating a low-zinc and high-zinc fraction, by utilizing the tornado process. The tornado is a high-velocity cyclone operating on pre-heated air, further details on the equipment can be found in the paper by Tikka et al.10) The freshly produced BF sludge contained 35.6% iron, 23.6% carbon and 0.40% zinc. The average iron, carbon and zinc content based on six samples of the low-zinc fraction of the BF sludge sampled during the operation of the tornado was determined to 38.1, 27.1 and 0.24%, respectively. A ThermoScientific ARL 9800 X-ray fluorescence (XRF) instrument with a rhodium tube was used to determine the iron content. A LECO CS444 combustion infrared detection analyzer was used for the determination of carbon. This analyzer can detect free carbon and carbon in carbonate material. Inductively coupled plasma sector field mass spectrometry (ICP-SFMS) was used to analyze the zinc content. The digestion of the sample prior to the ICP-SFMS analysis was achieved by microwave-assisted dissolution in a mixture of nitric acid, hydrochloric acid and hydrofluoric acid.
Three different recipes of cement-bonded briquettes, Table 1, were designed in the present study: i) a reference recipe without added BF sludge, ii) a recipe with 10 wt.% upgraded BF sludge labeled B1 and iii) a recipe with 20 wt.% upgraded BF sludge labeled B2. The reference briquette recipe was designed as a simplified version (omitting residues of small quantities) of the briquettes used in the full-scale operation at SSAB Luleå during the year of the pilot-plant scale trials. Before the briquetting, the materials were mixed in a forced action mixer with several impellers. A VU600/6 unit (developed by TEKSAM) operating on vibrating press technology was used to produce hexagonal briquettes with a height of 7 cm and an edge-to-edge width of 6.5 cm. The briquettes were cured in humidified atmosphere for 24 hours and then left in ambient room conditions for further curing during 27 days. The cold strength of the briquettes was determined by measuring the tumbling index (TI) after one day and four weeks of curing, respectively. These TI measurements were made using a modified version of ISO 3271 where the sieving was performed using a 6.0 mm sieve instead of a 6.3 mm.
| Material (wt.%) | Reference | B1 | B2 |
|---|---|---|---|
| Low-zinc fraction of BF sludge | 0.0 | 10.0 | 20.0 |
| Desulfurization scrap | 36.0 | 31.4 | 26.8 |
| BOF coarse sludge | 18.0 | 15.7 | 13.4 |
| BOF fine sludge | 12.0 | 10.5 | 8.9 |
| Briquette fines | 12.0 | 10.5 | 8.9 |
| BF dust | 10.0 | 10.0 | 10.0 |
| Cement | 12.0 | 12.0 | 12.0 |
The feasibility of top-charging the briquettes to the BF was evaluated by studying the high-temperature behavior during the descent in the campaign of autumn 2016 in the EBF. Hallin et al.11) presented a thorough description of the EBF. The briquettes were weighed and included in cylinder-shaped steel wire baskets. These baskets were charged in eight different coke layers where a briquette of each recipe was present in each layer. The baskets descended together with the burden material until the EBF was stopped and quenched with nitrogen gas. As the basket samples were charged in selected coke layers at the end of the campaign, the baskets were distributed throughout the vertical direction of the EBF shaft before quenching. After sufficient cooling, excavation of the EBF was carried out by carefully measuring, examining and photographing the different layers. During the excavation, baskets in six out of the eight layers were successfully recovered and analyzed.
The excavated briquettes were photographed and weighed. Representative samples of each briquette were collected after crushing and grinding the briquettes. A PANalytical Axios XRF instrument with a rhodium tube was used to determine the chemical composition of the briquettes. The metallic iron and ferrous iron contents were determined by titration using ISO 2597. Carbon in the briquettes was analyzed using the LECO as previously described.
The mineralogical composition of the briquettes was determined using a PANalytical Empyrean X-ray diffraction (XRD) unit operating on Cu Kα radiation. Selected briquettes were analyzed using scanning electron microscopy (SEM) together with energy dispersive X-ray spectroscopy (EDS). For this purpose, a Gemini Zeiss Merlin microscope with an Oxford EDS detector was used.
2.2. Industrial-scale BF Trials 2.2.1. Briquetting and BF TrialsEnough amount of upgraded BF sludge was not prepared for the industrial-scale trials by using the tornado treatment, as the treatment was performed only on technical scale. Thus, non-upgraded BF sludge was incorporated in the cold-bonded briquettes used in the industrial-scale trials to simulate the recycling of upgraded BF sludge in industrial-scale operation. The BF sludge used in the industrial-scale trials was prepared and briquetted by SSAB Merox in Luleå.
Drying of the BF sludge was required prior to briquetting, this was achieved by spreading the sludge on a prepared area open to the air. After approximately one year, the moisture content was down to 26 wt.%, which was acceptable for handling and briquetting. Briquettes produced based on two different recipes were studied: one corresponding to the reference briquette (RB) and one where part of the desulfurization scrap was substituted by 3.8 wt.% BF sludge (BF sludge briquette, BSB), Table 2. The recipes used for producing the briquettes for the industrial-scale trials were based on the available materials and their moisture contents. This explains why the recipes of Tables 1 and 2 differ. Table 3 shows the chemical composition of the briquettes produced from the five different recipes presented in Tables 1 and 2.
| Material (wt.%) | BSB | RB |
|---|---|---|
| Steel scrap | 10.0 | 10.0 |
| Desulfurization scrap | 22.5 | 26.3 |
| BOF fine sludge | 6.6 | 6.6 |
| BOF coarse sludge | 6.6 | 6.6 |
| Briquette fines | 20.6 | 20.6 |
| Mill scale | 2.0 | 2.0 |
| BF dust | 11.6 | 11.6 |
| BF sludge | 3.8 | 0.0 |
| Filter dust | 1.9 | 1.9 |
| Cement | 11.6 | 11.6 |
| Water | 2.9 | 2.9 |
| Element | BSB | RB | Reference | B1 | B2 |
|---|---|---|---|---|---|
| Fe | 36.2 | 37.3 | 50.6 | 51.5 | 52.8 |
| CaO | 24.3 | 23.2 | 19.9 | 17.9 | 16.5 |
| SiO2 | 7.9 | 8.0 | 4.6 | 5.0 | 5.1 |
| Al2O3 | 2.5 | 2.8 | 1.6 | 1.8 | 1.7 |
| MgO | 2.7 | 2.6 | 2.0 | 2.0 | 1.9 |
| MnO | 1.0 | 1.2 | 0.6 | 0.7 | 0.6 |
| C | 10.0 | 9.1 | 7.7 | 10.8 | 12.8 |
| S | 0.76 | 0.70 | 0.91 | 1.25 | 1.10 |
| Zn | 0.081 | 0.076 | 0.039 | 0.098 | 0.141 |
| K | 0.21 | 0.24 | 0.13 | 0.18 | 0.18 |
The briquettes were analyzed for the chemical composition using XRF and LECO combustion analysis. Furthermore, the cold strength of the briquettes was determined with the TI according to the tumbler test as previously described in section 2.1.1. The TI measurements were made after one day and three weeks of curing, respectively.
After curing, the BSB were charged during a trial period of three days to BF No. 3 at SSAB Luleå using an average charging rate at 97.3 kg per ton hot metal (tHM). A reference period with three days of stable operation was selected during which the RB were charged at an average rate of 99.6 kg/tHM.
The evaluation of the industrial-scale trials was conducted using operational data from the reference period and trial period. The process stability was evaluated considering values of, and variations in, parameters such as burden descent rate, top gas efficiency (EtaCO, Eq. (1)), hot metal quality and permeability. Generated amounts of sludge and dust and their compositions were used to assess the raw material efficiency. Furthermore, the raw material efficiency in terms of e.g. carbon efficiency was evaluated using heat and mass-balance calculations.
| (1) |
Heat and mass-balance calculations were used for evaluation of the industrial-scale trials. The calculations were made using a one-dimensional heat and mass balance model called Masmod.12) In the model, the BF process is divided into an upper and lower zone that are connected via the equilibrium between the gaseous components CO/CO2 and H2/H2O and the solid Fe/FeO in the thermal reserve zone. The input of materials to the lower zone of the model is the injectants and blast whereas the top-charged materials are added to the upper zone. Elemental and energy balances are made while taking into account the composition of inputs and outputs, sensible heat as well as reactions and measured heat losses. These balances are closed through iterative calculations minimizing the deviation between calculated and measured values, e.g., production rate, top-gas composition, slag amount and slag composition. Further details on the model have been provided by Hooey et al.12)
For the reference and trial period, the coke rate was calculated in Masmod with the assumption that the measured heat losses were accurate. The shaft efficiency and thermal reserve zone temperature were iterated by utilizing measured parameters such as the top-gas composition and temperature. The impact on top gas temperature, top gas efficiency and coke rate when changing composition of the briquettes charged to the BF was compared. In these calculations, the same oxidation degrees Eq. (2) of the RB and BSB were used as the distribution of the valences of iron was not analyzed.
| (2) |
The reference, B1 and B2 briquettes produced in technical scale, Table 1, were also considered in the calculations using Masmod. The results of these calculations were utilized to predict the effect of using briquettes with higher BF sludge content in the industrial-scale BF. As these briquettes were analyzed for the valences of iron and estimated for the carbonate content, the influence of the oxidation degree, carbonate and fixed carbon content on the results were considered.
Table 4 presents a summary of the different calculation cases used in Masmod. The first case was calculated using the data from the days of operation during which the RB were charged, i.e. the reference period. The data was used to close the balances where after this set could be used as a calibrated case for calculating other cases. Case 2 was calculated with the same operational conditions as Case 1; however, the composition of the BSB was used instead of the RB. Case 3 and 4 were evaluated considering the data from the trial period. The cross calculations of case 2 and 4, i.e. using BSB during the reference period and RB during the trial period, were performed to isolate effects corresponding to the operational conditions and briquettes. The operational conditions during the trial and reference period are presented in Table 5 illustrating that the conditions were quite similar in both periods. The reducing agent rate in Table 5 includes coke (and nut coke), pulverized coal injection (PCI) and BF dust injection. Cases 5, 6 and 7 were calculated by assuming the same operational conditions as during the reference period and replacing the RB with the reference, B1 and B2 briquettes, respectively. In the last three cases, the limestone addition was fixed to avoid the influence of the energy consumption from carbonate decomposition on the results. Instead, the slag basicity was controlled by BOF slag additions.
| Case | Time period | Briquette | Explanation |
|---|---|---|---|
| 1 | Reference | RB | Evaluation case for reference period |
| 2 | Reference | BSB | Simulation of reference period assuming use of BSB |
| 3 | Trial | BSB | Evaluation case for trial period |
| 4 | Trial | RB | Simulation of trial period assuming use of RB |
| 5 | Reference | Ref. | Simulation of reference period assuming use of Ref. |
| 6 | Reference | B1 | Simulation of reference period assuming use of B1 |
| 7 | Reference | B2 | Simulation of reference period assuming use of B2 |
| Parameter | Unit | Trial | Reference |
|---|---|---|---|
| Pellets | kg/tHM | 1345 | 1345 |
| Briquettes | kg/tHM | 97.3 | 99.6 |
| Reducing agent rate | kg/tHM | 451 | 449 |
| Slag rate | kg/tHM | 166 | 162 |
| Blast flow | kNm3/h | 243 | 246 |
| Specific blast volume | Nm3/tHM | 936 | 917 |
| Blast temperature | °C | 1099 | 1096 |
| Flame temperature | °C | 2163 | 2167 |
| Top gas flow | kNm3/h | 380 | 385 |
| Top gas temperature | °C | 114 | 135 |
| EtaCO | % | 56.4 | 56.3 |
| Direct reduction ratio | % | 32.2 | 31.8 |
| Permeability index | PV bosh | 2.40 | 2.35 |
| Hot metal quality | °C | 1438 | 1436 |
| Hot metal quality | %C | 4.71 | 4.60 |
| Hot metal quality | %Si | 0.35 | 0.34 |
| Hot metal quality | %S | 0.04 | 0.06 |
Figure 1 illustrates the vertical positions of the excavated baskets with respect to the outline of the EBF. All baskets were recovered below the upper probe and 1064 mm above the tuyere level.
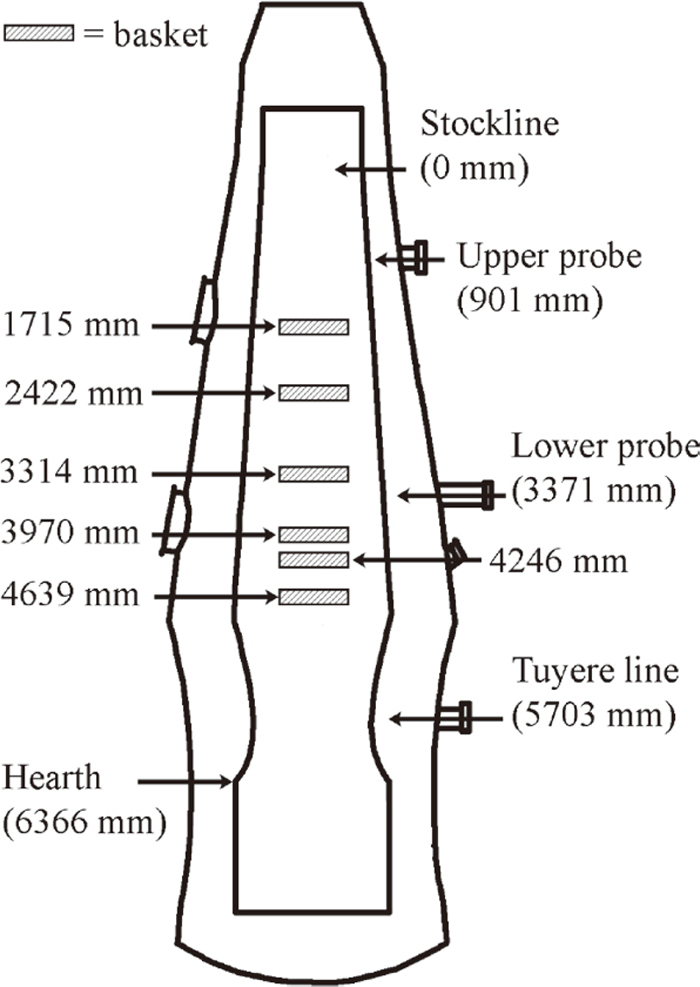
Vertical positions of the baskets in the EBF.
As the conditions may vary in the radial direction of the EBF, the orientation of the briquettes in the plane of each level is of interest. The horizontal positions of the baskets, labeled with the distance from the stockline, are illustrated in Fig. 2. The order of the three briquettes denoted in the figure as R, B1 and B2, where R denotes the reference briquette, represents the relative positions of the briquettes in the baskets.
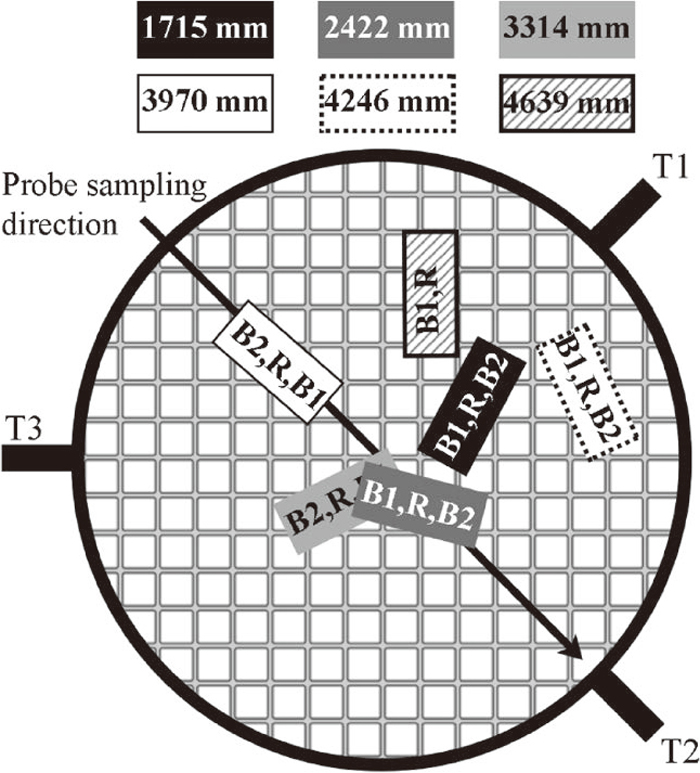
Horizontal position of the baskets in the EBF, T denotes tuyere.
The burden charging practice during the campaign aimed to allow a uniform gas distribution in the radial direction rather than operating with a central chimney. The variations in temperature and gas composition along the diameter, measured at nine different points by the upper probe, are illustrated in Fig. 3(a). The sampling direction of the upper and lower probe relative to the tuyeres and baskets is illustrated in Fig. 2. The measured gas temperature by the upper probe varied between 626 and 722°C. These values were lower than the actual gas temperature as the probe was water-cooled. Figure 4 presents the temperature measurements of the vertical probe, which was not water-cooled. The offset between the temperature measurements of the vertical probe and upper probe was determined to be 217–313°C. The path of the vertical probe cannot be controlled as the probe descend together with the burden. Thus, the horizontal position at each vertical position is unknown.
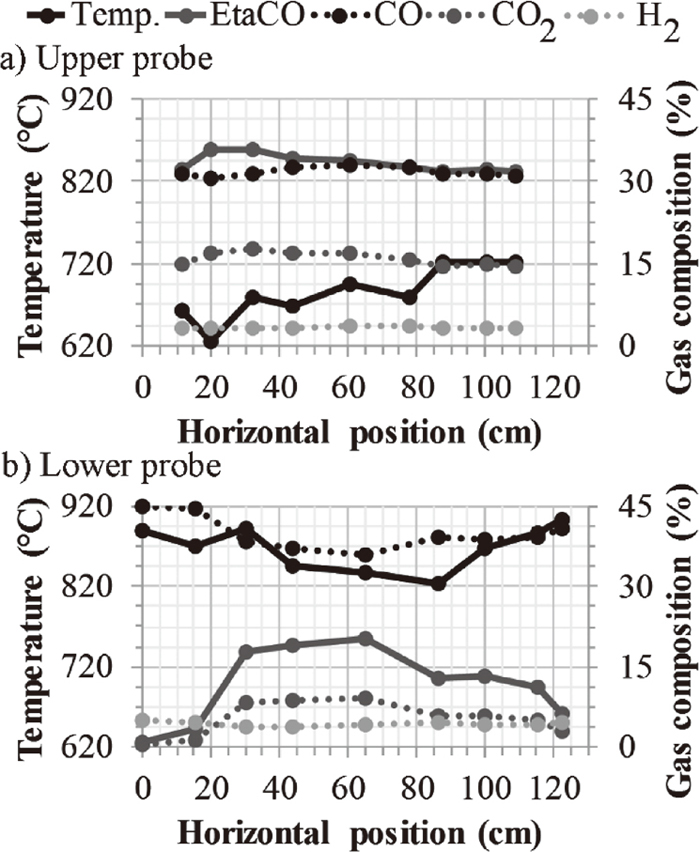
Probe measurements by a) upper probe and b) lower probe. Tuyere 2 is positioned on the right side of the x-axis.
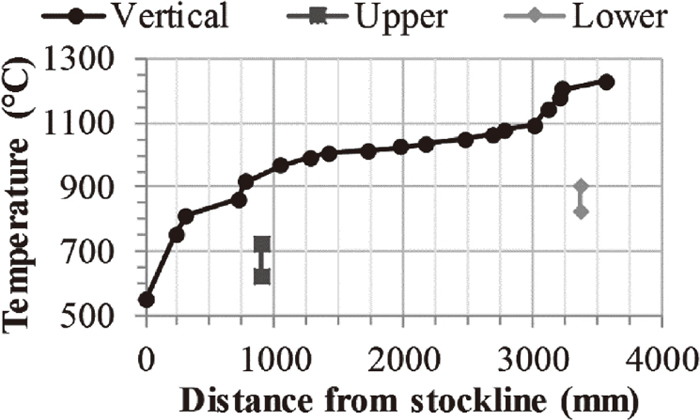
Vertical probe temperature measurements compared to the temperature measurements of the upper and lower probe.
The gas and temperature profile was also measured further down in the shaft by the lower probe Fig. 3(b). Again, nine different points located along the diameter were measured. The temperature varied between 823 and 904°C with the lower temperatures measured in the center of the furnace. The offset as compared to the vertical probe measurements at the same position was recorded to 310–391°C.
The higher temperatures on one side of the furnace wall and not the other, recorded by the upper probe measurements, Fig. 3(a), suggest that the gas had a preferred path along the wall. The temperature measurements of the lower probe, Fig. 3(b), suggest that the furnace was wall working, i.e. the central gas flow was impeded and the gas was preferentially flowing along the wall.5)
Considering the probe measurements, Fig. 3, and horizontal positon of the baskets, Fig. 2, the descent of each basket is unique in terms of the descent through different temperature and gas conditions.
3.1.2. Observations after the ExcavationMeasurements of the dimensions of the briquettes showed no swelling; instead, shrinking in one direction of the briquettes was indicated. This observation is in accordance with previous studies of cold-bonded briquettes in the EBF.13,14)
The briquettes in the three layers closest to the stockline were all intact after the excavation. The reference and B1 briquette recovered 3970 mm below the stockline were intact whereas the B2 briquette had a large piece chipped off from one of the corners. The reference and B1 briquette were broken at 4246 mm below stockline. However, they were partially intact at 4639 mm below the stockline. The part of the basket holding the B2 briquette was destroyed at 4639 mm below the stockline. Therefore, no information about this briquette could be retrieved.
During the material handling in conjunction with the crushing, grinding and sampling of the briquettes, no differences were observed in terms of how brittle they were. However, the pieces of the reference and B1 briquette found at 4246 mm below the stockline were observed to break easier than the intact B2 briquette from the same level.
Considering these observations, the briquettes containing the BF sludge were not compromised in terms of strength. These results are in line with the cold strength determined by the TI measurements after 28 days of curing. The TI of the reference, B1 and B2 briquettes was determined to 78, 84 and 81%, respectively.
Figure 5 illustrates the mass loss of the three different briquettes during the descent in the EBF. A rapid mass loss occurred prior to reaching 2422 mm below the stockline. After this, a zone of non-significant mass loss was observed before reaching the lower sections of the shaft where additional mass loss was observed. The chemical analyses and XRD measurements of the briquettes were used in order to relate the mass loss to the reactions occurring in the briquettes. The shaft of the EBF was roughly divided into an upper, middle and lower part, Fig. 5, each of which is considered in the sections below.
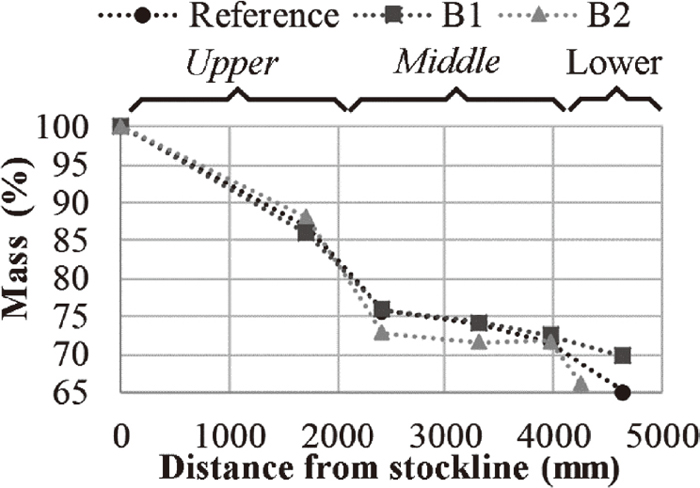
Mass loss during the descent in the EBF.
The diffractograms of the non-reduced B2 briquette and the B2 briquette recovered at 1715, 2422 and 4246 mm below the stockline are presented in Fig. 6. The diffractogram measured for the B2 briquette was chosen to be presented as this briquette had the highest content of Fe(III) and Fe(II) and lowest content of metallic iron before reduction, Fig. 7. Nonetheless, the non-reduced briquettes of the three different recipes had the same mineralogical composition on a qualitative level.
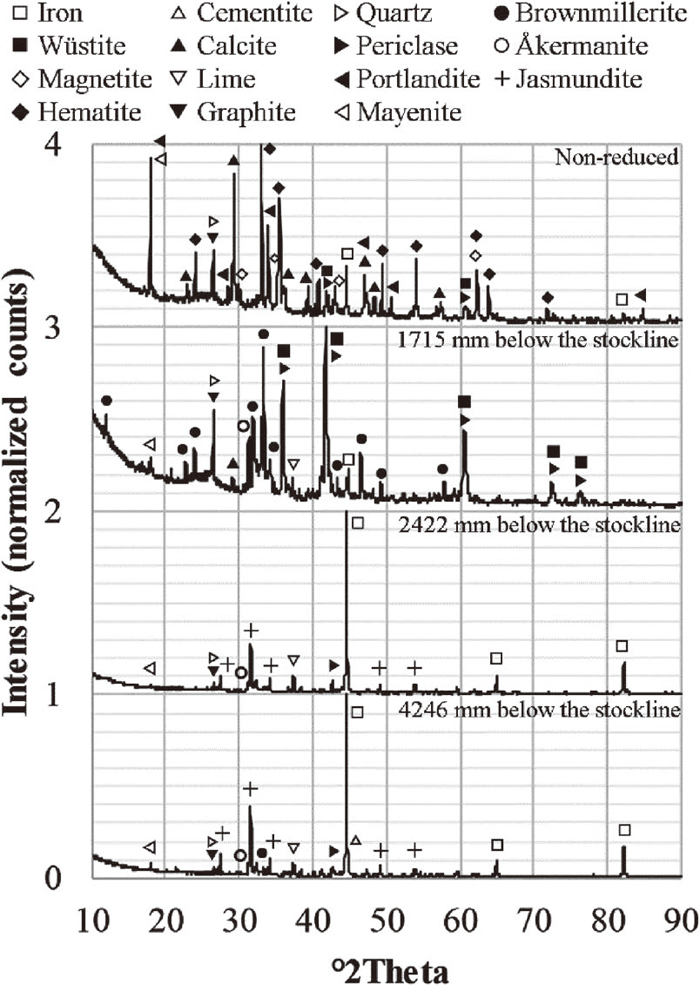
Diffractograms of selected B2 briquettes.
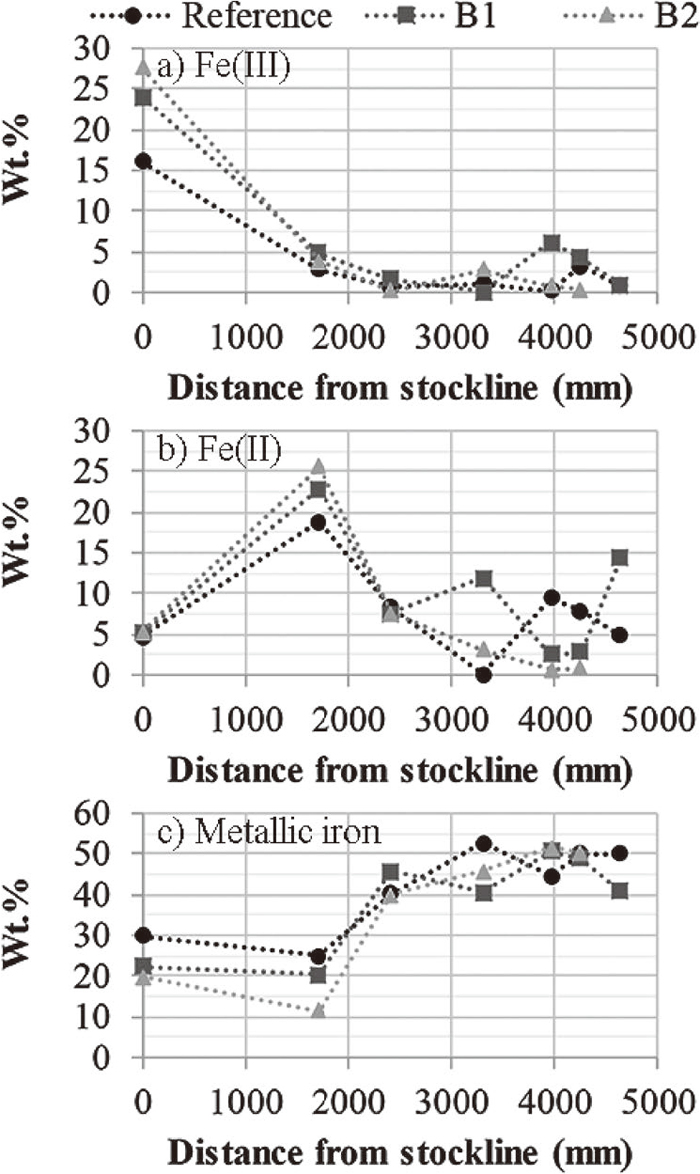
a) Fe(III) content, b) Fe(II) content and c) metallic iron content in the reference, B1 and B2 briquettes.
The metallic iron in the briquettes was found to be partly oxidized during the early descent in the EBF, reaching 1715 mm below the stockline, Fig. 7. The oxidation was also seen in the diffractograms, the second peak of iron detected at 82.8° 2 Theta was not detected at 1715 mm below the stockline, Fig. 6. This phenomenon has been observed in a previous publication where it was attributed to the solid diffusion of oxygen from higher oxidation states to lower ones.15)
The reduction of Fe(III) to Fe(II) is apparent considering the lower Fe(III) and higher Fe(II) content when comparing the non-reduced briquettes and the ones that reached 1715 mm below the stockline, Fig. 7. Furthermore, neither hematite (Fe2O3) nor magnetite (Fe3O4) were detected in the briquette at 1715 mm below the stockline, Fig 6. Also, the peak detected at 41.7° 2 Theta, shared by wüstite (FeO) and periclase (MgO), probably representing a solid solution of wüsite and periclase, was recorded with the highest intensity in the diffractogram, i.e., 100% relative intensity. This suggests that both the reactions in Eqs. (3) and (4) were completed. However, the valences of iron suggest that Fe(III) was present at 1715 mm below the stockline. This Fe(III) may be distributed in wüstite, brownmillerite (Ca2(Al,Fe)2O5) or possibly in magnetite present below the detection limit of the XRD. The Fe(III) content in wüstite can range from 7.5 to 29.9 wt.% of the total mass of wüstite.16)
| (3) |
| (4) |
The thermodynamic stability regions of magnetite, wüstite and iron corresponding to different temperatures and gas compositions are presented in Fig. 8. The diagram was reproduced using FactSage 7.1 utilizing the FactPS database. The calculations were made with a total pressure of two atmospheres representing the top pressure used during the EBF campaign. The measurements of the upper and lower probes are included in the figure. The upper probe measurements suggest that the reduction to iron is thermodynamically feasible prior to reaching 1715 mm below the stockline. However, considering the vertical probe measurements, shifting the gas temperature to the right in Fig. 8 illustrates that wüstite was the stable phase of iron in the conditions of the upper probe. These results are in line with the progression of the reduction.
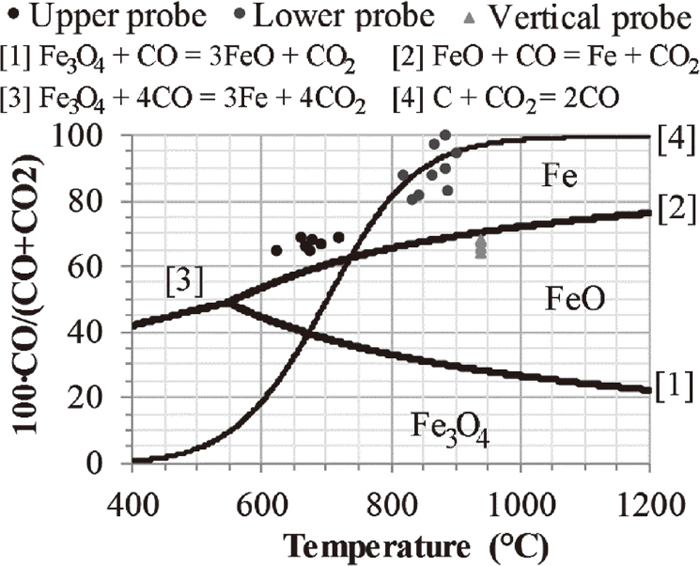
Fe–C–O equilibrium diagram at a total pressure of two atmospheres. Vertical probe measurements at 901 mm below the stockline.
Portlandite reacts to calcite in the presence of carbon dioxide according to Eq. (5). This reaction was finished prior to reaching 1715 mm below the stockline. Furthermore, brownmillerite and åkermanite (Ca2MgSiO7) were detected at 1715 mm below the stockline. In addition, the calcination reaction, Eq. (6), had started, which can be seen from the detection of lime (CaO) in the 1715 mm briquette and the drop in relative intensity and amount of peaks detected as calcite (CaCO3) when going from the non-reduced briquette down to the 1715 mm briquette. Also, the calcination is apparent considering the lower carbon content of the briquettes reaching 1715 mm below the stockline as compared to the non-reduced briquettes, Fig. 9.
| (5) |
| (6) |
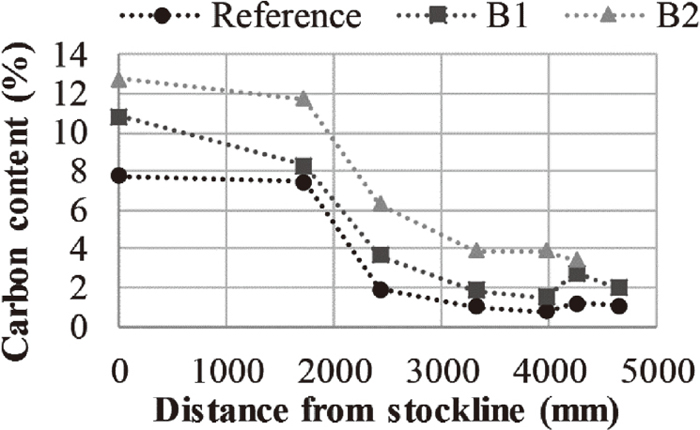
Carbon content of the briquettes.
Based on the above, the mass loss prior to reaching 1715 mm below the stockline, Fig. 5, can be attributed to the evaporation of free and crystal bound water, the reduction of the iron oxides and the partial completion of the calcination reaction.
3.1.4. Reactions in the Briquettes in the Middle Part of the ShaftThe temperature measurement of the vertical probe was determined to 1047°C at 2422 mm below the stockline. Considering that metallic iron was the major iron phase at this position, Fig. 6, the gas composition were within the stability region of metallic iron in Fig. 8. The detected periclase peaks in the diffractograms were shifted and did not share the same peak position with wüstite from 2422 mm below the stockline and downward in the EBF. However, the Fe(II) content, Fig. 7, suggest that a noticeable amount of ferrous iron was present at 2422 mm below the stockline. The reference briquette found at 3314 mm below the stockline was analyzed using SEM-EDS finding solid solutions of (Fe,Mg)O. This suggests that the reaction in Eq. (7) was not completed and that the ferrous iron was distributed in the solid solution of (Fe,Mg)O.
| (7) |
Considering Fig. 6, the calcination reaction had progressed to an extent where calcite was not detected in the briquettes reaching 2422 mm below the stockline. Thus, the descent down to this location was associated with the major part of the reduction as well as the complete calcination. Furthermore, the major change in the carbon content occurred during the descent down to 2422 mm below the stockline, Fig. 9. The change in the carbon content exceeded the loss in carbon related to the calcination reaction for carbonates suggesting that the carbon contributed in the reduction. Considering the gas composition at the lower probe (Fig. 3(b)) and the vertical probe temperature measurements at the position of the lower probe (Fig. 4), the direct reduction of wüstite, Eq. (8), was possible according to Fig. 8. In Eq. (8), the wüstite is not reduced by direct contact with coke; instead, the carbon dioxide of Eq. (7) reacts with the carbon in coke according to the Boudouard reaction in Eq. (9).
| (8) |
| (9) |
Based on the above, the mass loss going from 1715 mm to 2422 mm below the stockline, Fig. 5, was the continuation of the reduction and calcination as well as the contribution of carbon in the reduction. Furthermore, the completion of the calcination and the extensive propagation of the reduction explains the sluggish mass loss after 2422 mm below the stockline. Furthermore, the additional mass loss observed after this sluggish mass loss can be attributed to the partial breaking of the briquettes.
3.1.5. Reactions in the Briquettes in the Lower Part of the ShaftConsidering the previous section, the results suggested that parts of the briquettes were not completely reduced as Fe(II) in (Fe,Mg)O were detected using the SEM-EDS. In the lower part of the shaft, cementite (Fe3C) was formed in all briquettes suggesting low oxygen and high carbon monoxide partial pressures. Therefore, three different iron phases ((Fe,Mg)O, Fe and Fe3C) were present simultaneously in the briquettes. These results are in line with previous results from the EBF where more than two iron phases were present simultaneously during the descent due to the continuous reduction of the iron oxides at the agglomerate surface while the conditions within the agglomerate did not allow further reduction.17)
The degree of reduction (DoR) was calculated for each briquette in order to assess the effect on the addition of BF sludge on the reducibility. The DoR was based on the oxygen bound to iron, Eq. (10).
| (10) |
Considering Fig. 10, the B2 briquettes had consistently reached a higher reduction degree further down in the EBF. The same was not observed for the reference and B1 briquettes. Therefore, average values representing the DoR of the briquettes from 2422 mm below the stockline and downwards in the EBF were calculated. The average DoR was calculated to 72, 71 and 90% for the reference, B1 and B2 briquette, respectively. The C/O molar ratios of the reference, B1 and B2 briquettes before reduction were calculated to 0.92, 1.05 and 1.14, respectively. Increasing C/O molar ratios have been shown to facilitate an improved reduction degree18,19,20) and rate19) in self-reducing agglomerates. However, consistently increasing DoR with increasing C/O molar ratios was not observed, possibly, as the conditions vary locally in the EBF; e.g., the B2 briquettes were consistently facing towards the wall (Fig. 2) which the lower probe indicated had more reducing conditions (Fig. 3). Nonetheless, the lower average DoR of the B1 briquette as compared to the reference was considered acceptable. Thus, the addition of upgraded BF sludge up to 20 wt.%, as tested in the present study, was concluded not to have significant impact on the reducibility of the briquettes when charged to the EBF.
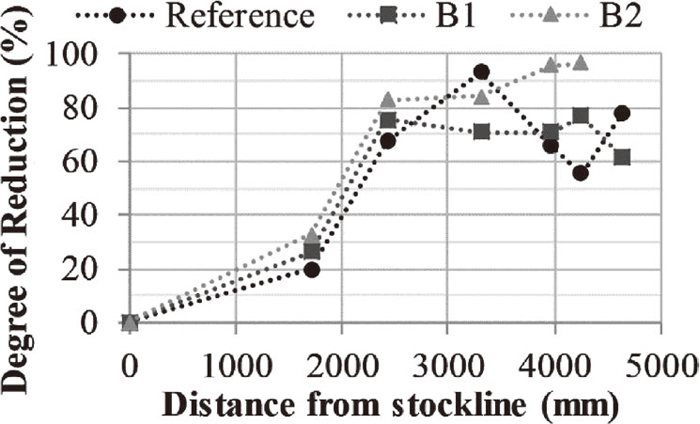
Degree of reduction of the briquettes in the vertical direction in the EBF.
Based on the above, the addition of upgraded BF sludge did not have significant effect on the strength or reducibility of the briquettes when charged to the EBF. Furthermore, as the EBF to a high extent represents the conditions in the industrial-scale BF operation,21) the promising results of the basket samples were considered of interest to verify in industrial-scale BF trials. For this purpose, cold-bonded briquettes with non-treated BF sludge were used as an approximation of the addition of upgraded sludge. Furthermore, the briquettes used in the industrial-scale trials had a lower amount of BF sludge as compared to the briquettes used in the basket samples charged to the EBF, compare Tables 1 and 2. Essentially, a different briquette was used, which required characterization prior to the top-charging in the industrial-scale BF.
3.2. Industrial-Scale BF Trials 3.2.1. Characterization of BriquettesThe chemical composition of the briquettes produced for the industrial-scale trials is presented in Table 3. The difference in carbon content between the RB and BSB was only about 10% due to a shortage of BF dust during the briquetting of the BSB; i.e., the content of BF dust in the BSB did not reach the expected level according to the designed recipe presented in Table 2. The difference in the chemical composition reflects the substitution of the iron-rich desulfurization scrap with BF sludge.
The cold strength after 21 days of curing of the RB, the first batch of the BSB and the second batch of the BSB, determined by the TI measurements, was 81, 74 and 68%, respectively. Thus, replacing desulfurization scrap by BF sludge decreased the cold strength. The difference in the cold strength measured for the first and second batch of the BSB can be attributed to the shortage of BF dust that arose during the briquetting process. Although both batches of the BSB had lower TI values, the required cold strength for top-charging in the BF at SSAB Luleå has previously been empirically determined to 60. Thus, the briquettes with BF sludge were considered suitable to charge in the industrial-scale BF trials.
3.2.2. Trial and Reference PeriodA planned maintenance stop in the steel plant occurred during the trial period, which resulted in a decreased production of hot metal (from 266 to 238 tHM/h) during approximately 14 hours. After the trial period, the operation with RB was performed subsequently for a few days as the reference period. After comparing data from the trial and reference periods and removing effects clearly linked to the decreased production, no disturbances could be attributed to the BSB. The product quality and process stability in terms of hot metal quality and its variation as well as variations in parameters such as gas efficiency and pressure drop was similar during the reference and trial period, Table 5. Furthermore, effects from fines generation in the shaft, e.g. in terms of lower permeability or disturbed descent connected to the lower TI values of the BSB as compared to the RB, were not observed.
The average sludge generation was higher during the trial period as compared to the reference period, as shown in Table 6. The dust generation peaked during the second day of the trial period, later receding to the same level as the first day. The peak value of the dust generation in the trial period is more likely due to the increased gas velocity above burden level (going from 1.10 to 1.16 m/s), observed during a six hour window of the decrease production, rather than the lower cold strength; especially as the dust generation reconciled to the same level as before. The higher gas velocity originated from a lowered oxygen enrichment (going from 4.5 to 1.74%) and therefore higher specific blast volumes (going from 911 to 971 Nm3/tHM) during the 14 hour period of decreased production. Furthermore, the dust generation was, on average, lower during the trial period as compared to the reference period suggesting that other factors than the TI value had impact on the dust generation.
| (kg/tHM) | Sludge | Dust | ||
|---|---|---|---|---|
| Day | Trial | Reference | Trial | Reference |
| 1 | 2.7 | 2.5 | 13.8 | 18.3 |
| 2 | 3.1 | 3.1 | 21.9 | 18.3 |
| 3 | 3.5 | 2.9 | 13.5 | 17.0 |
| Average | 3.1 | 2.8 | 16.4 | 17.9 |
The average zinc content in the BF sludge generated during the trial period was 0.58%, with a maximum of 0.80% during the second day. The average zinc content of the sludge during the reference period was determined to 0.40%. In addition, the average zinc content of the dust was determined to 0.21 and 0.05% during the trial and reference period, respectively. Thus, the average outlet of zinc via the top-gas was determined to 52.4 and 20.2 g/tHM during the trial and reference period, respectively. In contrast, the increase in zinc input by replacement of RB with BSB was only 3.1 g/tHM. An elevated outlet of zinc is normally expected after lowered production where after most of the increase in zinc outlet was not attributed to the increased zinc load of the BSB briquettes, especially as the maximum registered zinc content of 0.80% in the sludge coincided with the observed increased top gas temperature going from 109 to 130°C.
3.2.3. Reductant Rates in the Industrial-Scale TrialsThe results of cases one through four, defined in Table 4, are presented in Fig. 11. The reductant rates reproduced as coke equivalents, including carbon in the briquettes and injected dust, were similar in all cases. Based on the calculations, the extra carbon charged to the process when replacing the RB with the BSB substitutes approximately 1 kg of the coke per ton hot metal. However, this was calculated with the assumption that all briquettes had the same oxidation degree (Eq. (2)).
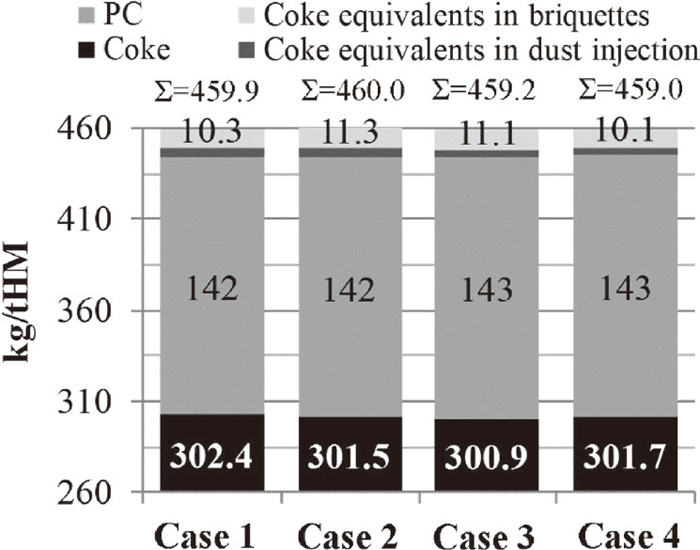
Calculation cases 1–4: consumption of coke, PC and coke equivalents from briquettes and BF dust injection.
Considering the results presented here and the previous section, the recycling of BF sludge to the industrial-scale BF via the cold-bonded briquettes was achieved without any negative effects. Based on the rate of addition of the cold-bonded briquettes and the annual production of hot metal from BF No. 3, 11.4 kton of upgraded BF sludge can be recycled via cold-bonded briquettes each year. This covers the annual on-site generation of BF sludge. Naturally, in the application of these results, the BF sludge has to be dezinced prior to recycling.
3.2.4. Potential for Higher Recycling RatesThe EBF trials indicated that more upgraded BF sludge could be recycled via the cold-bonded briquettes without affecting the performance of the briquettes in the process. This suggests that more BF sludge than the annual generation at BF No. 3 can be recycled. Thus, some previously landfilled sludge could be incorporated in the briquettes. As the briquettes produced for the EBF trials were analyzed for the valences of iron, a more accurate heat and mass balance could be determined, cases 5–7 in Table 4.
The wt.% of iron with different valences in the briquettes charged as basket samples to the EBF was used to calculate the oxidation degree and C/O molar ratio. The oxidation degree was calculated by using Eq. (2) to 0.57, 0.80 and 0.89 for the reference, B1 and B2 briquette, respectively. Although the briquettes with upgraded BF sludge had a higher oxidation degree, they also showed a higher self-reducing capability. As presented in section 3.1.5, the reference, B1 and B2 briquettes had C/O molar ratios of 0.92, 1.05 and 1.14, respectively. Thus, the briquettes containing upgraded BF sludge were completely self-reducible (C/O molar ratio equal to or higher than unity) whereas the reference briquette was not.
Calculating on a fixed production rate of hot metal, the higher fraction of metallic iron and lower oxidation degree charged to the BF using the reference briquettes requires less carbon for complete reduction as compared to the B1 and B2. This is illustrated in Fig. 12 where the reference briquette has the lowest overall reduction rate. Nonetheless, the carbon content in the upgraded BF sludge is high enough to reduce the additional iron oxides. Therefore, the coke consumption is not negatively affected when incorporating up to 20 wt.% of the sludge in the briquettes.
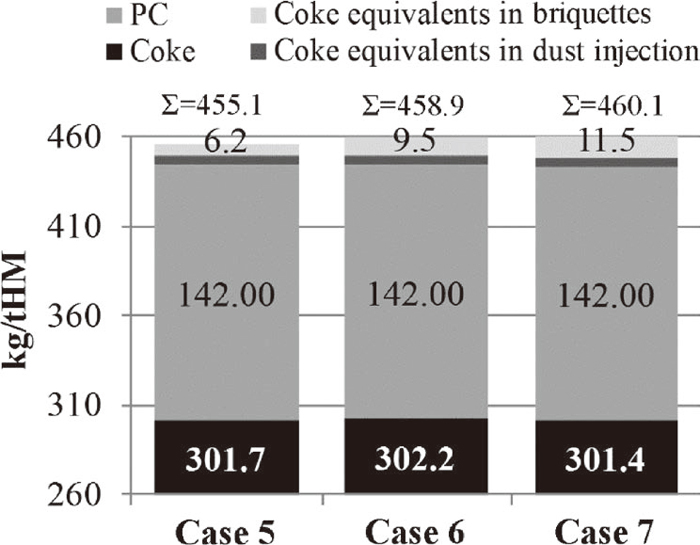
Calculation cases 5–7: consumption of coke, PC and coke equivalents from briquettes and BF dust injection.
Geerdes et al.5) presented a rule of thumb where charging 10 kg of scrap per tHM decreases the coke rate by 2–3 kg/tHM. In the present study, the desulfurization scrap mainly consists of metallic iron (approximately 60%). Referring to Table 1, the difference in scrap charged to the BF between the reference briquettes and B2 briquettes is about 9 kg/tHM more for the reference with a total charging rate of 99 kg of briquettes per tHM. According to the rule of thumb, this corresponds to a reduced coke rate of approximately 1.1–1.6 kg/tHM. In addition, Robinson22) characterized BOF coarse and fine sludge from the same site as the present study finding that the main iron phase is metallic iron. Therefore, the effect of the addition of upgraded BF sludge into the briquettes on the coke rate is even more pronounced as BOF coarse and fine sludge were replaced by the upgraded BF sludge. Considering this, the calculations should be made with a constant charging rate of metallic iron between the cases in order to study the effect of the upgraded BF sludge on the coke rate.
Table 7 summarizes important parameters for the three calculation cases considering charging of the briquettes produced using the recipes of Table 1 in the industrial-scale BF. The addition of 20 wt.% upgraded BF sludge in the briquettes was accompanied by a minor decrease in the required charging rate of iron ore pellets. Furthermore, the addition of upgraded BF sludge generated a more acidic briquette, which is reflected by the higher BOF slag addition and slag amount required to keep the B2 basicity constant at a value of one. The lower amount of metallic iron and higher amount of iron oxides charged to the process contribute to increased gas volumes. Consequently, the calculated gas flow rate and top gas temperature increased slightly.
| Parameter | Case 5 | Case 6 | Case 7 |
|---|---|---|---|
| Iron ore pellets (kg/tHM) | 1325 | 1325 | 1324 |
| BOF slag (kg/tHM) | 30 | 38 | 42 |
| Slag rate (kg/tHM) | 149 | 153 | 154 |
| Top gas flow (kNm3/h) | 372.3 | 375.5 | 376.7 |
| Top gas temperature (°C) | 134.6 | 135.9 | 136.5 |
| EtaCO (%) | 56.3 | 56.1 | 56.0 |
| Blast flow including moisture (kNm3/h) | 242.0 | 243.9 | 244.5 |
The addition of higher amounts of upgraded BF sludge is beneficial in terms of some parameters as presented above. Utilizing these higher recycling rates of BF sludge would allow landfilled sludge to be utilized. However, a carefully executed zinc balance over the integrated steel plant is required to evaluate if this way of recycling will generate an excessive load of zinc in the BF.
In the present paper, the recycling of BF sludge incorporated in cold-bonded briquettes has been studied in pilot-plant and industrial-scale BF trials. Briquettes produced based on three different recipes were studied in pilot-plant scale using the LKAB EBF: one reference briquette without added BF sludge, one briquette with 10 wt.% and one with 20 wt.% of the low-zinc fraction of tornado-treated BF sludge. Based on the pilot-plant scale experiments, adding upgraded BF sludge to the briquette resulted in:
• A briquette capable of withstanding the reduction under load inside the EBF.
• A briquette with proper reducibility that was left satisfactorily reduced in the lower part of the EBF shaft.
• A briquette that can be used to recycle upgraded BF sludge to the BF.
Cold-bonded briquettes with and without pre-dried BF sludge was charged at a rate of approximately 100 kg/tHM to BF No. 3 at SSAB Luleå concluding that:
• The lowered cold strength accompanied with the added BF sludge did not affect the stability of the BF operation.
• 11.4 kton of upgraded BF sludge can be recycled annually to BF No. 3, assuming that the BF sludge in the cold-bonded briquette can be replaced by the low-zinc fraction of tornado-treated BF sludge.
The authors wish to thank the Swedish Energy Agency and the research program Iron and Steel Industry Energy Use (JoSEn) for financial support. The authors would also like to thank the project committee members of JK21069 Smart Recycling of Residues from Ore Based Steelmaking for their feedback. The project was conducted within CAMM – Centre of Advanced Mining and Metallurgy at Luleå University of Technology. The comments on the manuscript provided by Professor Bo Björkman is acknowledged.