2019 Volume 59 Issue 10 Pages 1893-1900
2019 Volume 59 Issue 10 Pages 1893-1900
The microstructural evolution and microhardness changes of the H13 ingot during homogenization process were investigated to characterize the dendrite segregation changes by optical microscopy (OM), scanning electron microscopy (SEM), electronic probe micro-analyzer (EPMA), X-Ray diffraction (XRD) and microhardness testing. The results showed that the severe dendrite segregation existed in H13 ingot, and there were a large amount of coarse non-equilibrium eutectic carbides (MC and M7C3) in dendrite region. After the soaking time of 15 h at 1200°C, the dendrites almost disappeared, the contents of eutectic carbides decreased sharply. The values of segregation ratio SR were 1.20 for Cr, 1.26 for Mo and 1.41 for V, which were the critical values of SR that marked the end of homogenization. After 20 h, a small amount of MC carbides still distributed at the grain boundaries. The SR values almost remained constant with the values of 1.10, 1.14 and 1.14 for Cr, Mo and V element respectively. The homogenization kinetics was established and it matched well with the experimental data. The microhardess in different soaking time reflected well the dendrite segregation changes, it decreased from 817.8 HV to 645.7 HV and then increases to 752.5 HV, finally it decreases gradually.
The Cr–Mo–V steel, AISI H13, is belonged to hypereutectoid steel with about 8 wt.% alloying elements and 0.4 wt.% carbon. It is widely used throughout the world at present as hot-working dies, which is applied in traditional thermal deformation process of metallic material. Its operating temperature is up to 600°C, which is quite closed to the subsequent tempering temperature.1,2) Excellent high temperature strength, impact toughness, hot cracking resistance and thermal fatigue of H13 are required.3) Researches4,5) have shown that, to a great extent, there has the relation between the resistance to heat checking and impact toughness. Moreover, excellent impact toughness in transversal direction is a significant feature for H13 in NADCA criterion.1)
However, due to solute redistribution of the alloying elements during solidification process, whose partition coefficient is less than 1, the initial solidified region promotes dendrites arms, containing low concentration of alloying elements and carbon. As the solidification proceeds, alloying elements and carbon continuously segregate out from the dendritic crystal, as a result, the chromium, molybdenum, vanadium and other alloying elements enrich in grain boundary regions or interdendritic regions. Once the concentration of alloying elements attains the eutectic point, simultaneously satisfying the thermodynamic and kinetic conditions, these alloying elements will form larger eutectic carbides accompanied with inclusions sometimes.4,6) The most important ones would be M6C, MC, M7C3 and M23C6 in H13 ingot, reported by Wang7) and M. R. Ghomashchi.8) The dendrite segregation and eutectic carbides will deteriorate hot workability and usability of H13 steel, especially damaging impact toughness eventually.4,9,10) So it is significant to study how to eliminate the inhomogeneity. Many scholars had done some experimental research to eliminate the inhomogeneity by high temperature homogenization treatment. M. Torkar et al.11) showed that at 1300°C the dendritic microstructure disappeared when the soaking time was shorter than 4 h, and at 1250°C a soaking time of between 4 h and 8 h was necessary owing to the slower diffusion process. Ma et al.4) showed that utilizing high temperature diffusion treatment (at 1200°C, holding for 8 h) of ingot prior to hot forging, the isotropy ratio of H13 steel was obviously improved, the segregation was nearly eliminated, and the as-annealed microstructure was more uniform. Lu et al.12) indicated in his study that high temperature homogenization treatment before forging at 1250°C for 16 h would increase the impact energy to 23 J. Accordingly, it can be efficient to eliminate the segregation to improve the final properties by high temperature homogenization treatment. Nevertheless, they simply took the high temperature homogenization treatment as a method to eliminate the segregation among these articles, there was no comprehensive and deep understanding about the degree of homogenization, the detailed changes during homogenization process were not described, such as crystal structure,13) grain size14) and segregation changes.
In general, it used to utilizing SR (Segregation Ratio) to characterize the uniformity of alloying elements distribution across the secondary dendrite arms. The microsegregation coefficient SR15) is defined as Cmax/Cmin, where Cmax represents the maximum concentration and Cmin the minimum concentration of the element. According to the mathematical model,16) if SR = 1, t → ∞. Obviously, it is not realistic in the industrial production, just a kind of ideal state. It is easily accepted that there is a critical value of SR that presents the steady and final state of segregation and the dissipation degree of primary carbide, which is the signal of the limit of soaking temperature and time. If the soaking temperature and time were extended continuously, the deterioration of mechanical properties for forging will take place. Previous study by Liu13) had proposed the view that when dendrites or dendritic structure disappeared at a given segregation ratio SRm, it means the termination of heat diffusion. This investigation was implemented to characterize the dendrite segregation changes of alloying elements Cr, Mo and V in as-cast H13 steel by studying the evolution of microstructure and microhardness during homogenization process, aiming to achieve the critical segregation ratio to guide the homogenization process before forging. The macro-segregations in the ingot were not considered.
In this study, the test steel was melted in an electric arc furnace and casted at 1450°C in 75 kg ingots in the form of 390 mm long billets with a diameter of 160 mm, The chemical composition was as followed (wt%): 0.42% C, 0.98% Si, 0.47% Mn, 0.95% V, 5.32% Cr, 1.25% Mo, 0.0031% P, 0.0028% S, 0.0033% N.
A cross-sectional plate with 20 mm thickness was cut from the ingot and used for metallographic observations of the as-cast microstructure. The equilibrium relation between carbides and temperature was calculated by Thermo-calc thermodynamic software based on database TCFE 9, which suggested that the total dissolved temperature of eutectic carbides was 1130°C in Fig. 1. Considering the nonequilibrium of microstructure, the temperature range for these carbides should be largely extended due to the element segregation. Homogenization trials were performed at 1200°C with soaking time from 0 to 30 h, cubic specimens of 20 mm × 20 mm × 20 mm for homogenization were machined from the region in the middle of the billet radius, avoiding the variations in the composition near the billet surface. The metallographic specimens were grinded, polished and then etched with 4% nitric acid, the microstructural morphology and the distribution of alloying elements were analyzed by optical microscopy (OM) and scanning electron microscopy (SEM), the area and line scanning examination were conducted on an electronic probe micro-analyzer (EPMA). Specimens for X-ray diffraction (XRD) were also prepared by standard mechanical polishing, followed by electrochemical polishing in a solution of 10% HClO4 in alcoholic at 20 V for 18 s. The XRD tests were conducted using a Cu-target in the scanning range of from 30° to 90° with the speed of 2°/min.
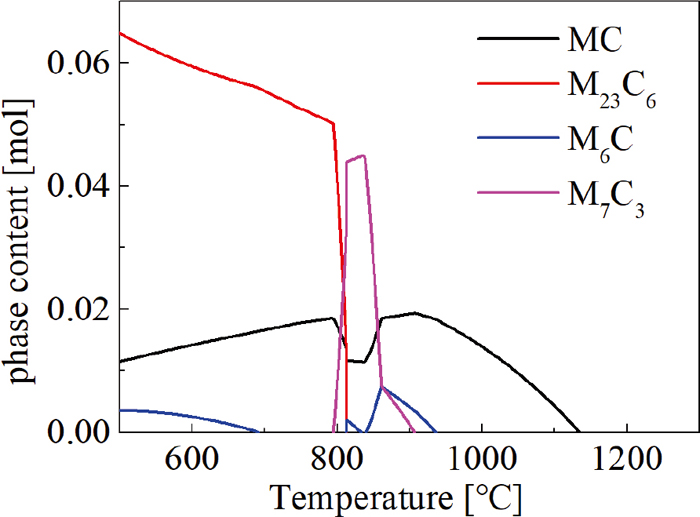
Calculation results of carbides of tested ingot. (Online version in color.)
The casting conditions that high cooling rate and non-equilibrium solidification result in the formation of non-equilibrium eutectic carbides and the heterogeneity of alloying elements.17) Optical micrographs on overall microstructure of ingot from surface to center are shown in Fig. 2. The structure of as-cast H13 consists of martensite and retained austenite with extensive interdendritic segregation, coarse carbides, and shrinkage voids, which result from the cooling rate. The overall as-cast microstructure presents the atypical dendritic morphology, exhibiting severe dendrite segregation, on which consists of typical eutectic structure. Moreover, from surface to center of ingot the degree of segregation is more and more serious [Figs. 2(a), 2(b), 2(c)], and the initial grain size increases corresponding to dendritic arm spacing. The strip area with gray color is the boundary of the dendritic crystal, in which the concentration of alloying elements is richer than those in the matrix due to the segregation during solidification. The black line is the grain boundary. Prior austenite grain boundaries are observed in the solute-rich interdendritic region, but are obscured in the more heavily etched solute-poor dendrite arms.18) The interdendritic region shows a net-shaped microstructure, in which chemical elements with partition coefficient less than 1 are concentrated and it presents a considerable number of coarse continuous non-equilibrium eutectic phases, which greatly deteriorates the strength and toughness of the alloy and brings some bad influences to the processing property and application [Fig. 2(d)].
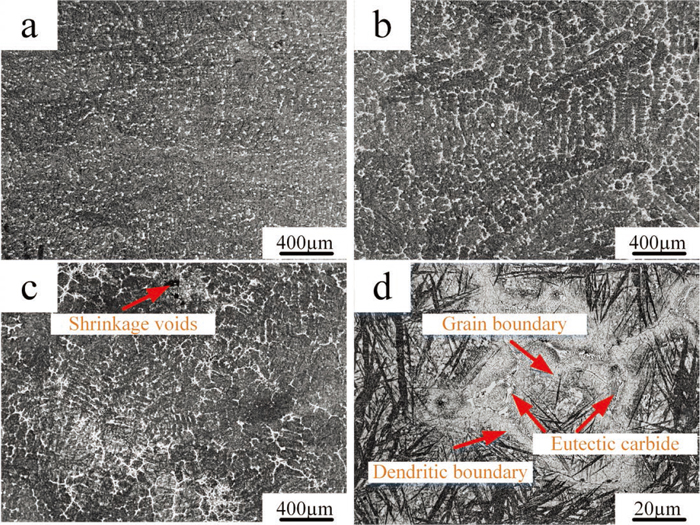
Metallographic images at different position of ingot: (a) surface; (b) half the radius; (c) center and (d) center at higher magnification. (Online version in color.)
The typical morphology of carbides of as-cast microstructure under SEM is shown in Fig. 3. There exist three different states in carbides: long-rod shape, fishbone shape, chained shape in as-cast microstructure, whose size could be even up to tens of microns. The results of EDS analysis of point A, B and C are listed in Table 1. The qualitative and quantified analyses indicate that the chemical composition of grey eutectic carbides [Figs. 3(a), 3(b), point A, B] is V-rich and that of bright phase [Fig. 3(b), point C] is Mo-rich. In Fig. 3(c), the image in the bottom right corner is the enlargement of region in white box, the small carbides distributed throughout the dendrite, they are formed in two morphologies, chains and cubes. In fact, the amount of V-rich carbides is far more than that rich in Mo in the as-cast ingot.7) The carbides type based on the component analysis can be characterized as follows:8)
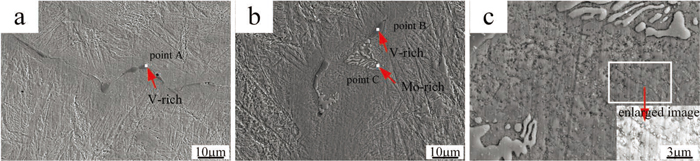
Morphologies of carbides in tested ingot. (Online version in color.)
| Carbide | Point | C | Cr | Mo | V | Mn | Fe |
|---|---|---|---|---|---|---|---|
| MC | A | 8.45 | 9.1 | 33.24 | 46.21 | 0.13 | 2.88 |
| B | 8.95 | 7.04 | 19.37 | 58.52 | 0.15 | 5.85 | |
| M7C3 | C | 5.06 | 18.11 | 38.5 | 10.44 | 0.65 | 26.99 |
Figure 4 is main micro-alloying element distribution maps of as-cast H13 steel under EPMA. It can be clearly seen that micro-alloying elements (Cr, Mo, V) concentration is larger in grain boundary than inside of grains, which is the same with Fe, proving the existence of carbide of (Fe, M)x Cy class19) (where M represents Cr, V and Mo substituting for Fe). The element segregation seriously deteriorates the properties of the alloys. Therefore, the high temperature homogenization treatment is necessary to eliminate the segregation of these elements in the as-cast alloy steel.
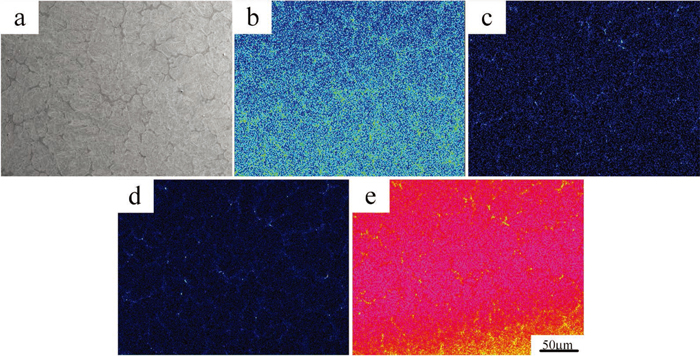
SEM microstructure and elements distribution of tested ingot: (a) SEM image; (b) Cr distribution; (c) Mo distribution; (d) V distribution and (e) Fe distribution. (Online version in color.)
Alloying element distribution can be reflected by the existence of the dendrites, namely that the dendrites eliminate or not can be one of the indications of high temperature diffusion process. The optical microstructure of homogenized specimens for different soaking time at 1200°C is shown in Fig. 5. It can obviously detect that there still exists distinct dendrite when the soaking time is 10 h, furthermore, the difference of interdendritic brightness decreases and the number of primary dendrite decreases with the prolonging of soaking time [Figs. 5(a), 5(b)]. With the soaking time reaching 13 h, the morphology of dendrite has been unable to identify and almost disappears [Fig. 5(c)]. The dendrites disappear completely and the microstructure of host material is clearly visible at 15 h and 20 h [Figs. 5(d), 5(e)], whose grain size is uniform. The phenomenon of mixed crystal occurs at 30 h [Fig. 5(f)].

Optical microstructure of tested specimens at 1200°C for different soaking time: (a) 5 h; (b) 10 h; (c) 13 h; (d) 15 h; (e) 20 h and (f) 30 h.
The morphologies of interdendritic primary carbides at different soaking time are listed in Fig. 6. The primary carbides become smaller and sparser with prolonging soaking time visually. Besides, the morphology of Mo-rich carbides (M7C3) make the changes from fishbone shape [Fig. 6(a)] to discontinuous shape [Fig. 6(b)], and to point [Fig. 6(c)], which can be seen more clearly in the enlarged image of the white box. When the soaking time increases to 20 h [Fig. 6(d)], the Mo-rich carbides are almost dissolved into the matrix. The V-rich carbides (MC) still exist at 20 h, but the size and quantity decrease significantly. In addition, the microstructure of host material around the primary carbides is more distinct in the same corrosion method by the increasing of soaking time, which is also confirmed the alloying element segregation is eliminated. The XRD analysis is used to characterize the carbide content and type changes in heating process. As plotted in Fig. 7, the carbide peak features have no changes until 5 h. But after holding for 10 h the peak of M7C3 and M6C almost disappear, the number of MC peak decreases, which is the representation for the dissolution of carbides.
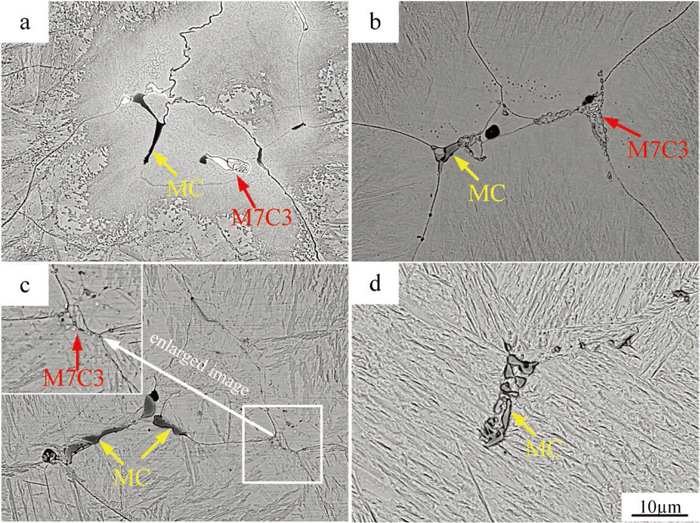
Evolution of primary carbides at 1200°C for different soaking time: (a) 0 h; (b) 5 h; (c) 10 h and (d) 20 h. (Online version in color.)
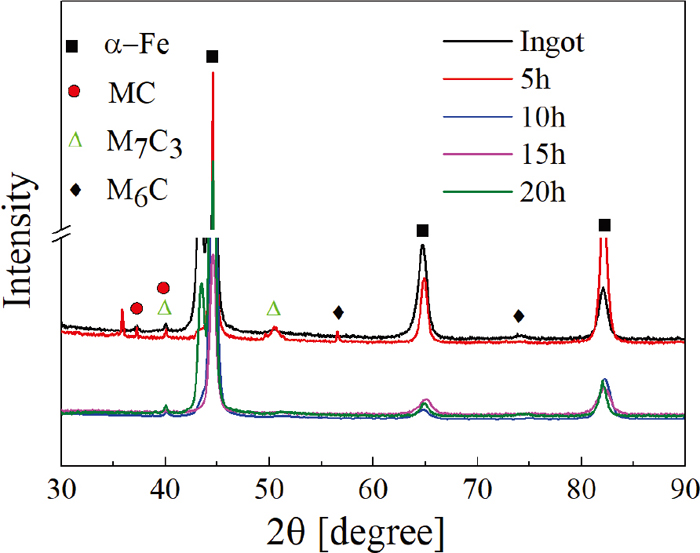
XRD measurements of the tested ingot at 1200°C for different soaking time. (Online version in color.)
As we know, grain growth would occur at elevated temperature for holding long time, and the presence of carbides on the grain boundary would weaken the trend. Specially, the diffusion speed of alloying elements would be fast at the grain boundaries, where it has higher distortion energy. The bigger the grain size is, the smaller the proportion of grain boundary is, the longer the diffusion process of alloying elements is.20) Moreover, the good state of grain boundary is favourable to subsequent forging process and the final properties.21,22) The coarse austenite grains after homogenization at 1200°C for 0 h, 5 h, 10 h, 15 h and 20 h are listed in Fig. 8. The prior austenite grain sizes distribute in a wide range of 10–240 μm, and the smaller grains are always surrounded by larger grains. It is obvious that the grain sizes in as-cast condition are uniform. With the prolonging of holding time, the grain sizes are larger, the difference in grain sizes are larger too. The alloying elements segregate into the grain boundaries and often form carbides. Austenite grain boundaries will be pinned by carbide particles, retarding their motion during reheating, which is discussed and testified by some researchers.22,23,24) In this study, carbides are found on austenite grain boundaries and may act as a barrier to the austenite growth, as indicated by Fig. 9, and the effective size for austenite grain boundary pinning is thought as 50 nm.14) Due to the presence of segregation region, the average carbide size is micron size in as-cast condition. So the carbide pinning effect on the austenite growth is not obvious at first, the eutectic carbides decompose and transform into the small particles with the prolonging of holding time, the pinning effect strengthens.
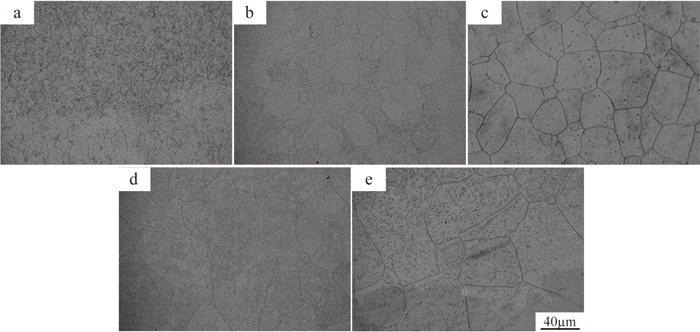
Austenite grains at 1200°C: (a) 0 h; (b) 5 h; (c) 10 h; (d) 15 h and (e) 20 h.
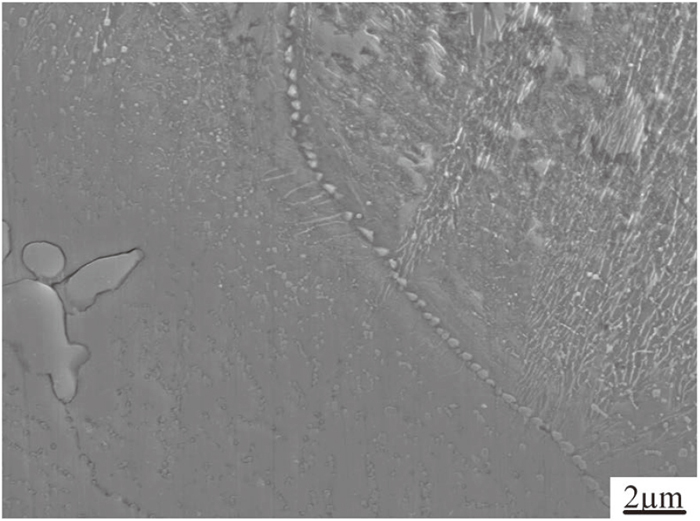
Carbide particles on austenite grain boundary at 1200°C for 10 h.
In order to characterize the improvement of dendrite segregation adequately, we introduce a concept of SR. Table 2 shows the results of SR in different soaking time by EDS line scanning, where the scanning line stretches over the second dendrite, but deliberately avoids the carbide area. From the values of SR, we can infer that the degree of segregation of V element is the most serious. By prolonging the soaking time, the values of SR decrease and have no obvious change when the soaking time is over 15 h. Moreover, they almost keep in a certain value approximately in the range from 20 h to 30 h, which can be powerfully demonstrated by the result of EDS line at 1200°C for 20 h in Fig. 10. Besides, considering the continued heat preservation at 1200°C after 20 h, grains will coarsen, which will deteriorate the properties of material.
| Soaking time/h | SR | ||
|---|---|---|---|
| Cr | Mo | V | |
| 0 | 2.07 | 3.09 | 4.37 |
| 2 | 1.88 | 2.67 | 3.87 |
| 5 | 1.72 | 2.22 | 3.08 |
| 8 | 1.53 | 1.79 | 2.42 |
| 10 | 1.4 | 1.34 | 2 |
| 13 | 1.29 | 1.29 | 1.73 |
| 15 | 1.2 | 1.26 | 1.41 |
| 18 | 1.16 | 1.17 | 1.2 |
| 20 | 1.1 | 1.14 | 1.08 |
| 30 | 1.09 | 1.1 | 1.07 |
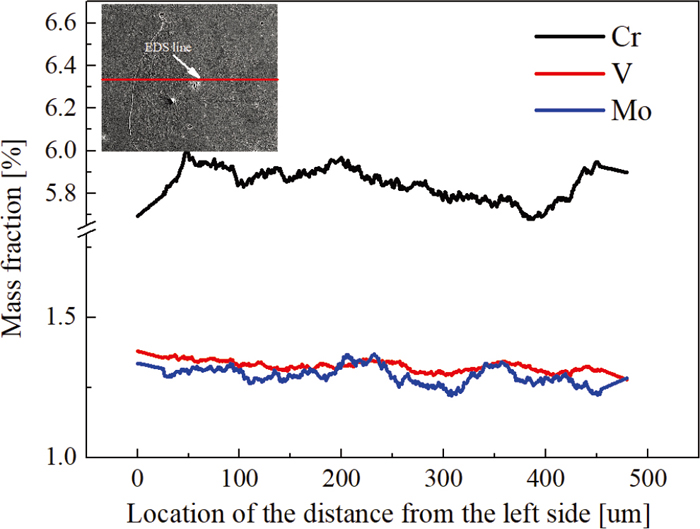
Results of EDS line scanning at 1200°C for 20 h. (Online version in color.)
As a result, the specific process parameters of experimental H13 ingot can be concluded (1200°C - 15 h). However, such parameters are only applicable to the experimental ingot. As the ingot size changes, the above parameters will no longer be applicable due to the change of secondary dendritic arm spacing L and initial segregation ratio SR0. Consequently, the process parameters obtained by structure observation alone lack universal applicability and cannot be extended to more ingots. Here, as the statement in the study of Liu,13) the critical segregation ratio SRm is determined by the disappearance of dendritic crystal.
At soaking time of 15 h, dendritic crystal disappear, numerous eutectic carbides dissolve and transform into small particles and austenite grain sizes almost keep constant between 15 h and 20 h, the segregation ratio SR of Cr element could be taken as the critical segregation ratio SRm. Therefore, the SRm is determined to be 1.20, namely, for H13 ingot of any size, high temperature diffusion can be terminated as long as the condition of SR ≤ 1.20 for Cr element is met.
3.3. Homogenization KineticsResearches16) had reported that the alloying elements present the cosine or sine distribution among interdendritic regions. So the key of investigating the distribution of the alloying elements during homogenization is to study the diffusion law along adjacent interdendritic region. The diffusion kinetic behavior of alloying elements during homogenization process can be analyzed in theory, according to the theory of Shewman and Fick’s diffusion law, many scholars had deduced the homogenization kinetic equation:
Where, A = R/Q, B = 4.6/4π2D0, R is the mole gas constant, 8.314 J/(mol·K), Q is the diffusion activation energy, D0 is the diffusion constant; L is the interdendritic spacing; T and t are the homogenization temperature K and time s, respectively.
Here, the documentation process of homogenization kinetic equation can be referred to many literatures,25,26) the homogenization kinetic curve can be plotted if the parameters of as-cast microstructure are given. Table 3 shows the alloying elements diffusion constant D0 and diffusion activation energy Q in γ-Fe matrix.27,28) What is more, the element diffusion speed is controlled by diffusion coefficient D on the basis of Fick’s diffusion law,29) which will be restricted by the alloying element with the minimum D value. Whilst the diffusion coefficient D at different temperature for alloying elements can be deduced by means of Arrhenius Equation, D = D0exp(−Q/RT). Table 3 indicates the diffusivity of V lies between that of Cr and Mo. Figure 11 shows the D value of Cr and Mo at different temperature, we can see that the D value of Cr is distinctly less than that of Mo, so it considers that the homogenization process will finish as soon as the concentration of Cr is uniform.
| Units | Cr | Mo | V | |
|---|---|---|---|---|
| Diffusion coefficient D0 | cm2/sec | 10.8 | 0.068 | 0.25 |
| activation energy Q | KJ/mol | 291.8 | 247 | 264.2 |

Diffusion coefficients at different temperature. (Online version in color.)
The homogenization kinetic curves of H13 steel can be obtained by substitution of D0, Q and R into homogenization kinetic equation, as shown in Fig. 12. The soaking time reduces greatly with the increase of homogenization temperature and with the decrease of interdendritic spacing. By quantitative metallographic analysis, the interdendritic spacing varies about from 133 μm to 200 μm over the same region and the primary dendrite arm spacing remains constant. At the experimental temperature of 1200°C, the corresponding desired soaking time is from 12 h to 31 h, as are indicated by the grey box, which is in good agreement with the experimental results.
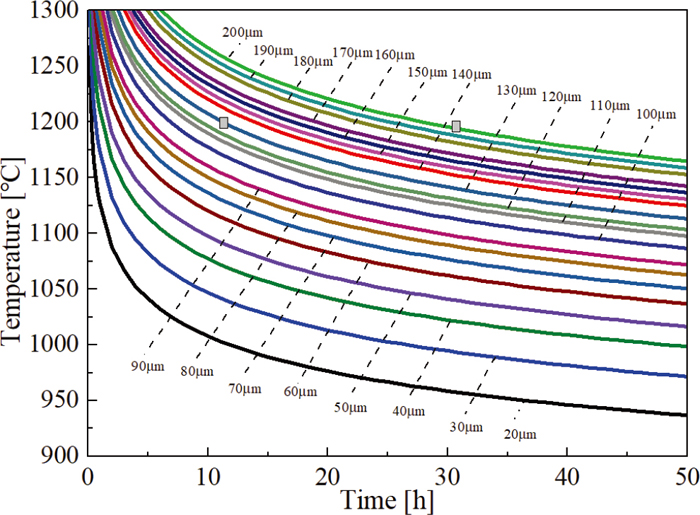
Curves of homogenization kinetics for Cr element. (Online version in color.)
As mentioned above, high temperature homogenization treatment can affect the microstructure obviously, especially the dendrite segregation and eutectic carbide. Meantime, microhardness is sensitive to the microstructure evolution, which is mainly attributed to the solid solution strengthening mechanism by segregated alloying elements. Figure 13 illustrates the average microhardness values variation of homogenized specimens during homogenization process. On the whole, it indicates that the microhardness values present the declining trend with the increasing of soaking time. The microhardness value after homogenization first decreases and then picks up with the extension of soaking time, the peak value appears at 10 h, but the peak value is lower than the microhardness value at the as-cast state. When to continue, the microhardness curve shows a declining trend too.
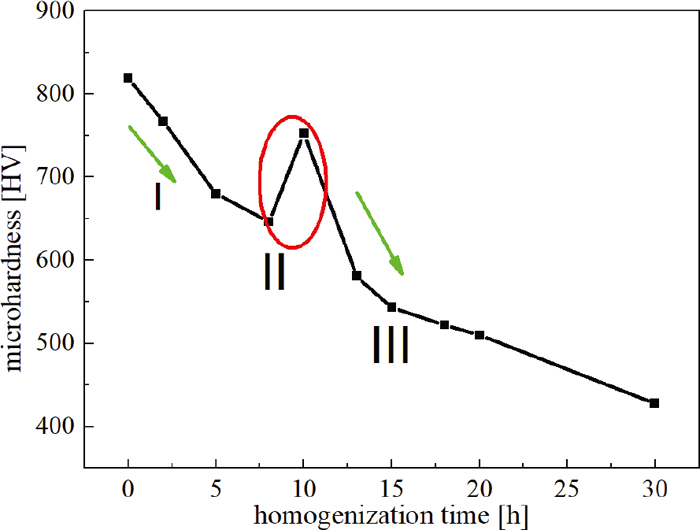
Microhardness distribution curve of the specimens after homogenization. (Online version in color.)
The variation of microhardness value can be divided into three regions I, II and III: the microhardness of as-cast H13 steel is very high, which is mainly related to those of supersaturated solid solution, precipitated phase and dislocation. In the early stage of homogenization, the elimination of residual stress and dislocation in the as-cast structure are the main cause of the decline in microhardness in the zone I. With the extension of soaking time, the atom’s kinetic energy increases, the coarse primary carbides gradually dissolve in the matrix and the interdendritic segregated alloying atoms motion to the adjacent dendritic arm region, the alloying element concentration difference becomes smaller in dendritic region, the effect of solid solution strengthening is predominant. Therefore, the average matrix microhardness value increases in zone II. But as soaking time goes on after 10 h in zone III, grain coarsening is more predominant, especially at high temperature, the microhardness value decreases continuously. Meanwhile, the microhardness value variation is a little between 15 h and 20 h by contrast with that between 10 h and 15 h, which can be supported by small changes of segregation ratio and grain size in section 3.2.
(1) The dendrite segregation widely distributed in H13 ingot, the main micro-alloying elements Cr, Mo, V were largely enriched around grain boundaries, the primary carbides were MC and M7C3 type.
(2) Increasing the soaking time at 1200°C, it resulted in a reduced amount of dendrite segregation and eutectic carbides in the H13 ingot, the dendritic structure disappeared at about 15 h, the stability of eutectic carbides MC was better than that of M7C3, the former still remained a certain amount and the later almost disappeared after the preservation of 20 h at 1200°C. The small carbide particles transformed by the large eutectic carbides pinned the grain boundary. The microhardess change during homogenization process included three stages, it decreased at first, affected by the elimination of residual stress and dislocation. Secondly, its increase depended on the solution strengthening owing to diffusion of segregation elements. At last, it was a steady downward trend owing to grain coarsening.
(3) The SR of micro-alloying element V was the highest in segregation region. The value of SRm (critical segregation ratio) was determined to be 1.20 for Cr element, standing for the termination of the homogenization process for H13 ingot in any size when the value of SR for Cr was less than 1.20.
(4) Based on the homogenization kinetic model for Cr element, the optimized homogenization processing for H13 steel was 1200°C - (12 h–31 h), which was consistent with the results of homogenization experimental study. The model was capable for the reference of the homogenization completion times in industrial scale.
This work was supported by the National Key Research Project of China (2016YFB0300402).