2019 Volume 59 Issue 5 Pages 805-809
2019 Volume 59 Issue 5 Pages 805-809
Experimental phase equilibria studies have been carried out to investigate the phase chemistry in the CaO–“Fe2O3”–SiO2 system related to the iron ore sinter making in 1 atm CO2 at 1200–1230°C. The measurements have been performed using a modified experimental technique involving high temperature equilibration and rapid quenching followed by electron probe X-ray microanalysis (EPMA). In contrast to the equilibria in air, the experimental results show that no silico-ferrite of calcium (SFC solid solution) is formed in 1 atm CO2 within the temperature range investigated; that is, the SFC phase is not stable at liquidus temperatures. The liquidus for the iron-rich corner of the CaO–“Fe2O3”–SiO2 system in CO2 atmosphere for the spinel (magnetite Fe3O4) and dicalcium silicate (C2S = Ca2SiO4) primary phase fields has been proposed.
Experimental measurements of the phase equilibria of the pseudo-ternary system CaO–“Fe2O3”–SiO2 in air in the iron-rich corner of the system were reported previously.1) It was shown that under those conditions the silico-ferrite of calcium solid solution (SFC phase2,3,4)) was stable at the liquidus temperatures in this system from 1192 to 1257°C. These results are of particular significance to the sintering of iron ores3,5,6,7,8,9,10) since they establish for the first time the conditions for the formation of SFC solid solution from the melt.
In industrial iron ore sinter making processes, coke is added to the initial (green) sinter blend and its combustion provides heat for the process. Limestone (CaCO3) is also added to enable calcination and incorporation of this flux into sinter prior to the charging into the blast furnace, and to provide CaO for the formation of the SFC phase and the CaO required for the blast furnace hearth slag. Limestone (CaCO3) decomposes on heating with the release of CO2. Hence, during the sinter-making process, the local oxygen partial pressure is expected to be lower than that of air (0.21 atm). It is essential to understand the effect of the gas atmosphere on the phase chemistry of iron ore sinters in order to be able to improve the control of the phases and microstructures formed in the sinter process, and ultimately improve sinter quality and blast furnace performance.
The effects of oxygen partial pressure on phase equilibria in the CaO–“Fe2O3”–SiO2 system relevant to the iron ore sinter-making at selected temperatures between 1240 to 1300°C (1513 K to 1573 K) have previously been studied experimentally.11) In those earlier studies mixtures of synthetic chemical reagents were equilibrated at oxygen partial pressure of 0.005 atm (i.e. logPO2 = −2.3); obtained by introducing a flow of N2–O2 gas mixture of total 1 atm with 0.5 vol.% O2. The samples were heated at fixed temperature for 24 hours and then quenched into liquid nitrogen. The samples then were mounted in resin block and polished for examination by optical microscope. Selected samples were measured by the electron probe X-ray microanalysis (EPMA) to determine the chemical compositions of the solid phases present at temperature. The SFC phase was found to form at 1240°C (1513 K) while no SFC phase was detected at other temperatures investigated, namely 1255°C, 1270°C and 1300°C.
In the present study, further detailed phase equilibria studies have been carried out; the difficulties associated with the quenching of the low-SiO2 liquid encountered by previous researchers have been overcome by developing an open sample support method, which has been described previously by the authors.1) The present investigation is focused on the effect of CO2 atmosphere on the formation of the SFC phase and liquid phases in the range of bulk compositions and process conditions of direct relevance to the iron ore sinter-making.
The experimental methodology used in the present study has been described in detail previously.1) This principally involves high temperature equilibration of synthetic chemical mixtures in selected gas atmospheres targeting two or more condensed equilibrium phases at the desired temperature, followed by direct quenching into iced water. The cross-sections of the samples were then prepared and examined using standard metallographic techniques. The phase compositions at equilibrium were measured by electron probe X-ray microanalysis (EPMA). The rapid quenching technique successfully retains the phase assemblages and phase compositions that are present in the sample at the equilibration temperatures. The compositions of various phases were measured using JEOL 8200L EPMA with wavelength dispersive detectors (JEOL is a trademark of Japan Electron Optics Ltd., Tokyo). A 15 kV accelerating voltage and 15 nA probe current were selected for the microanalyser operation. The standards (Charles M. Taylor, Stanford, CA) used in the EPMA measurements were as follows: wollastonite (CaSiO3) for Ca and Si, and hematite (Fe2O3) for Fe. The Duncumb–Philibert correction based on atomic number, absorption, and fluorescence (ZAF correction, supplied by JEOL) was applied. The accuracy of compositions measured was estimated to be within 1 wt%. Only the metal cations concentrations were measured with EPMA and the compositions were recalculated using selected oxidation states for presentation purposes. An additional correction for cation ratios has been established using Fe2SiO4, Ca2Fe2O5, CaFe2O4, and CaFe4O7 standards prepared in the laboratory.1)
No directly measured local oxygen partial pressures in the sinter bed during the iron ore sinter-making process were found in the literature. It was assumed that the gas composition during the sinter formation may vary between that in air (PO2 = 0.21 atm), to the products of complete combustion of carbon during sinter-making, i.e. 0.21 atm CO2 + 0.79 atm N2. The equivalent oxygen partial pressures at PCO2=0.21 atm and PCO2=1 atm (present study) at temperature range investigated are given in Table 1. From the Fe–O equilibria12) spinel (magnetite Fe3O4) is the stable pure iron oxide phase in this range of PO2 and temperatures. High purity CO2 (99.995%) supplied from COREGAS, Australia was used to create gas atmosphere for the present study. According to results of thermodynamic calculations shown in Fig. 1, this purity is sufficient to maintain the stable effective oxygen partial pressure, although care should be taken to avoid air leaks into the furnace. Another source of uncertainty in p(O2) is the initial excess of Fe3+ in the sample, that needs significant time of equilibration to be removed.
Equilibration experiments were undertaken in the CaO–“Fe2O3”–SiO2 system at 1 atm CO2 at temperatures of 1200°C, 1210°C, 1220°C and 1230°C (1473 K, 1483 K, 1493 K and 1503 K). The compositions of the phases measured in the present study are reported in Table 2. The following phases were observed: spinel Sp (Fe3O4-rich solid solution); dicalcium silicate C2S (2CaO·SiO2); rankinite C3S2 (3CaO·2SiO2); calcium monoferrite CF (CaO·Fe2O3) and liquid oxide solution of variable compositions. Examples of typical microstructures in the equilibrated samples are shown in Fig. 2. Figure 2(a) shows the microstructure of a well equilibrated, three-condensed phase assemblage of spinel – dicalcium silicate – liquid; Fig. 2(b) shows spinel and liquid in equilibrium. No SFC phase was observed in any the samples equilibrated in the range of conditions investigated.
| Temperature (°C) | LogPO2 (atm) | Experiment No. | Phases | Composition (wt%) | ||
|---|---|---|---|---|---|---|
| CaO | Fe2O3 | SiO2 | ||||
| 1200 | −3.87 | 1 | L | 25.6 | 69.6 | 4.8 |
| Sp | 8.9 | 90.4 | 0.7 | |||
| 2 | L | 26.0 | 68.6 | 5.4 | ||
| Sp | 10.3 | 89.5 | 0.2 | |||
| C2S | 64.7 | 1.6 | 33.7 | |||
| 3 | Sp | 3.7 | 95.2 | 1.0 | ||
| C3S2 | 57.8 | 1.9 | 40.3 | |||
| 1210 | −3.82 | 4 | L | 22.7 | 72.6 | 4.7 |
| Sp | 5.8 | 94.2 | 0.0 | |||
| 5 | L | 22.8 | 73.6 | 3.5 | ||
| Sp | 6.1 | 93.8 | 0.1 | |||
| 6 | L | 24.4 | 70.1 | 5.5 | ||
| Sp | 4.7 | 95.3 | 0.0 | |||
| C2S | 64.2 | 1.6 | 34.2 | |||
| 7 | Sp | 2.9 | 96.9 | 0.2 | ||
| C3S2 | 58.0 | 1.4 | 40.6 | |||
| 1220 | −3.78 | 8 | L | 22.8 | 74.2 | 3.0 |
| Sp | 5.3 | 94.1 | 0.7 | |||
| 9 | L | 23.3 | 72.6 | 4.0 | ||
| Sp | 4.8 | 94.7 | 0.5 | |||
| 10 | L | 24.4 | 69.9 | 5.7 | ||
| Sp | 4.4 | 94.7 | 0.8 | |||
| 1230 | −3.73 | 11 | L | 22.8 | 72.6 | 4.6 |
| Sp | 6.0 | 94.0 | 0.0 | |||
| 12 | L | 23.6 | 70.5 | 5.9 | ||
| Sp | 5.3 | 94.6 | 0.1 | |||
| 13 | L | 24.6 | 67.2 | 8.2 | ||
| Sp | 4.2 | 95.8 | 0.0 | |||
| 14 | L | 23.0 | 69.9 | 7.0 | ||
| Sp | 4.8 | 95.2 | 0.0 | |||
| 15 | L | 23.8 | 68.7 | 7.4 | ||
| Sp | 4.5 | 95.5 | 0.0 | |||
| 16 | L | 23.6 | 68.8 | 7.6 | ||
| Sp | 4.4 | 95.5 | 0.1 | |||
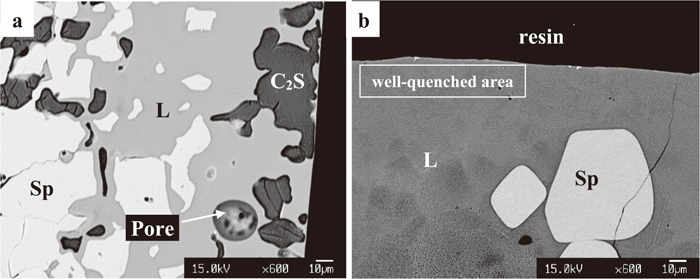
Examples of the microstructures observed (in backscattered electron mode) in the CaO–“Fe2O3”–SiO2 system in 1 atm CO2. a) spinel – dicalcium silicate – liquid equilibrium 1220°C (1493 K), b) spinel – liquid equilibrium 1220°C (1493 K). Legend: Sp = spinel, L = liquid, C2S = dicalcium silicate.
Using the data provided in Table 2, and the phase equilibria in the CaO–“Fe2O3” system predicted using the FactSage model,13,14,15,16,17) the liquidus for the CaO–“Fe2O3”–SiO2 system at 1 atm CO2 has been constructed and is shown in Fig. 3, including comparison to the liquidus in air.1)
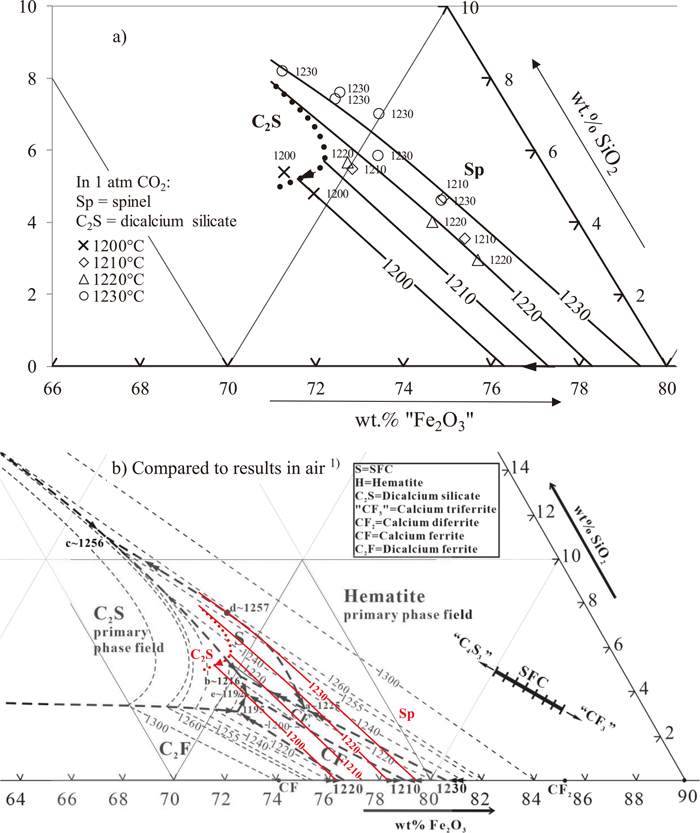
(a) Proposed liquidus of the CaO–“Fe2O3”–SiO2 system in CO2 (1 atm) based on experimental data obtained in the present study; (b) comparison to the previous study in air1) and FactSage 6.213,14,15,16) model predictions. Temperatures in °C. Thin dashed lines are estimated liquidus isotherms in air;1) thin solid lines are measured liquidus isotherms in 1 atm CO2; thick dashed lines are estimated univariant lines in air;1) thick dotted lines are estimated univariant lines in 1 atm CO2.
Note that spinel is the stable solid iron oxide under these conditions. It can be seen that there is no SFC primary phase field appearing at the liquidus temperatures. The shape of the dicalcium silicate boundary with spinel is based on predictions by FactSage using FToxid database13,14,15,16) under the conditions used in the present study.
Phase equilibria study results obtained by Pownceby et al.11) indicates SFC phase is stable up to 1240°C (1513 K) at PO2 = 5×10−2 atm. In the present study, no SFC phase was found between 1200°C and 1240°C (1473 K and 1513 K). It should be noted that the effective PO2 used in present study is between 10−3.9 to 10−3.7 atm, which is lower than that used in the previous study.11)
Figure 4 presents projection of the liquidus at fixed SiO2 concentrations.
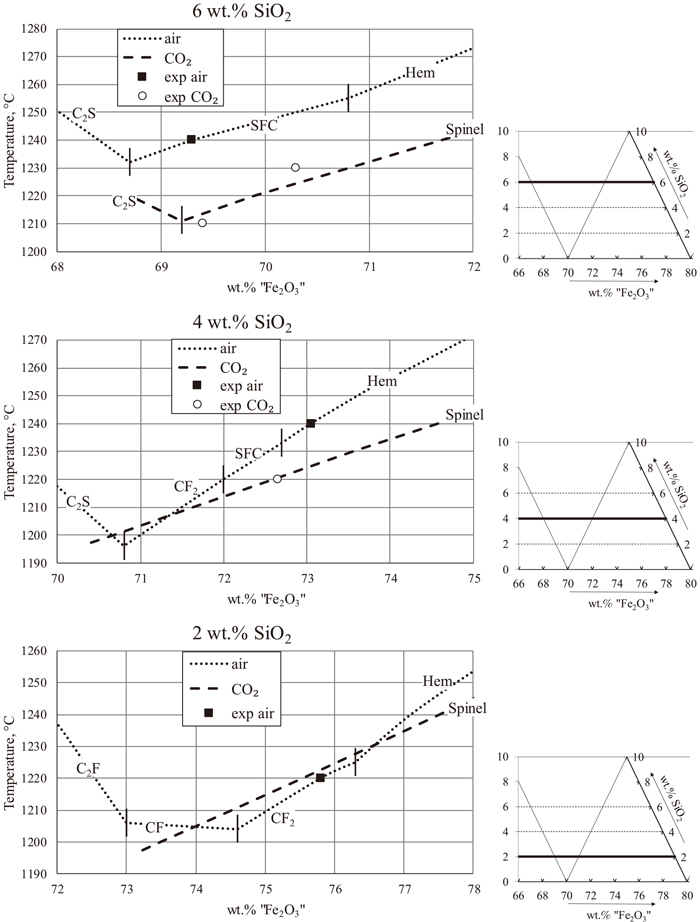
Projection of liquidus of the CaO–“Fe2O3”–SiO2 system in CO2 and air at fixed wt%SiO2 based on experimental data obtained in the present study and previous study in air.1) Legend: H = hematite, Sp = spinel, SFC = SFC solid solution, CF2 = calcium diferrite, C2S = dicalcium silicate.
The CaO concentration in the spinel in equilibrium with CO2 gas measured in the present study is significant, in the range of 4.5–10.5 wt.% CaO depending on temperature and liquid composition. The CaO distribution between spinel and liquid in the CaO–“Fe2O3”–SiO2 system at low SiO2 concentrations at 1 atm CO2 is given in Fig. 5.
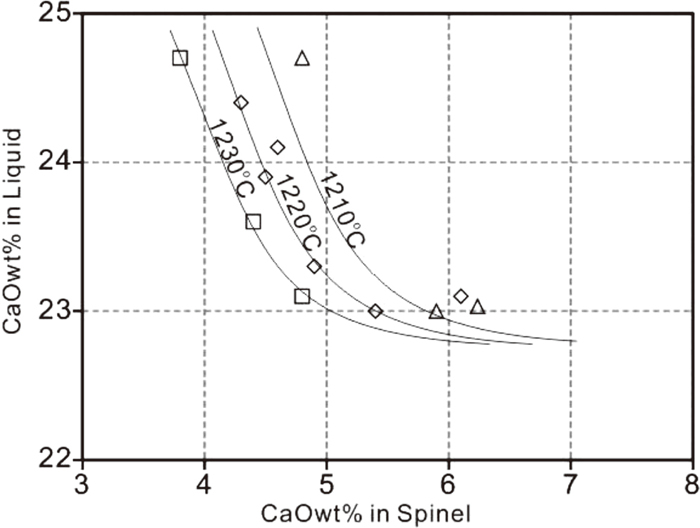
CaO distribution between spinel and liquid in the CaO–“Fe2O3”–SiO2 system at low SiO2 concentrations and 1 atm CO2 (Note no SFC phase present).
Solubility of SiO2 in the spinel phase up to 1 wt% has been measured in the present study; similar values were also found in previous studies.18,19) Figure 6 shows the solubility of SiO2 in the spinel phase in equilibrium with liquid in the atmosphere of 1 atm CO in the temperature range investigated. The solubility of SiO2 in spinel increases with the increase of SiO2 concentration in the liquid phase and the decrease of temperature.
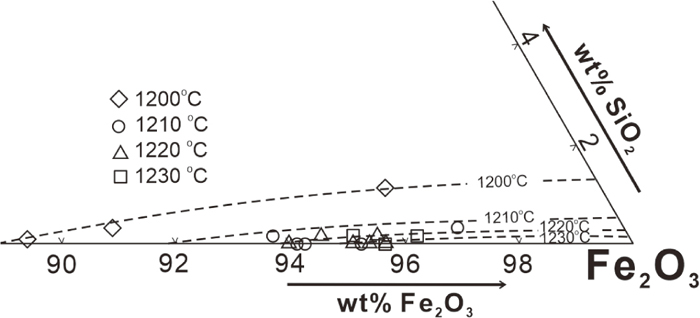
Solubility of SiO2 in spinel phase as a function of SiO2 wt.% in liquid at equilibrium with 1 atm CO2.
Phase equilibria studies have been carried out to investigate the thermodynamic stability of silico-ferrite of calcium (SFC) phase at equilibrium in 1 atm CO2. The experimental results show that no SFC phase is formed in 1 atm CO2 within the temperature range investigated. The liquidus surface for the CFS system in CO2 is therefore quite different to that obtained in air. The spinel (magnetite) primary phase field has replaced the hematite primary phase field as the dominant feature of the iron oxide-rich corner of the CaO–FeOx–SiO2 diagram and there is no primary phase field for SFC.
This research was supported financially by the Baosteel Australia Joint Research Centre (BAJC). The authors wish to thank the members of the Baosteel Ironmaking Institute for their collaboration; in particular the Director, Xiaoming Mao and Mr Qi Wei.
The authors would also like to thank to the staff of the University of Queensland Centre for Microanalysis and Microscopy (CMM) for their support in maintenance and operation of scanning and electron microprobe facilities in the Centre.