2019 Volume 59 Issue 8 Pages 1396-1403
2019 Volume 59 Issue 8 Pages 1396-1403
In order to produce high-strength coke from low-quality coals, noncovalent bonds between O-functional groups in coal were cleaved by pyridine containing HPC pyridine soluble and HPC-derived thermoplastic components were introduced into the pores formed by swelling; thus, the synergistic effect during carbonization of the suppression of cross-linking reactions and the fluidity amplification due to close placement of coal and thermoplastic components was investigated. When HPC was extracted with pyridine, a decrease in O-functional groups was observed in the pyridine-soluble and pyridine-insoluble components. When HPC was extracted with MeOH, on the other hand, O-functional groups in HPC selectively moved into the soluble components. When non- or slightly-caking coal was chemically-modified with the prepared HPC pyridine-soluble components by utilizing the solvent-swelling effect of pyridine, the fluidity improved compared with the coals physically mixed with the soluble components or HPC. On the other hand, the fluidity of the chemically-modified sample with the MeOH-soluble components hardly changed from that of the original sample, and no effect of the modification with the thermoplastic component was observed. Furthermore, it was clarified that higher-strength coke can be produced from the chemically-modified sample with the HPC pyridine-soluble components than from the original coal or the physically mixed coal with the soluble components. The contraction behavior during carbonization of the chemically-modified sample with the soluble components and that of the original coal was investigated; as a result, a large difference was not observed between these two. Thus, it was found that high-strength coke can be produced from low-quality coals by the present method.
For iron production, a further increase in the coke strength is awaited to reduce CO2 discharge by operating a blast furnace at a low reducing agent rate. However, the usage of high-quality caking coal, which is a raw material, has been increasing with the recent increase of steel demand in Asian countries. Thus, there are growing concerns about soaring prices and depletion of high-quality caking coals. Accordingly, from the viewpoint of the expansion of coal resources for coke production, the technology development for the coke production from cheap, abundant, low-quality coals [slightly-caking coal and non-caking coal (subbituminous coal, etc.)] is important.
In recent years, a coke production method, in which the usage of caking coal can be reduced by adding coal solvent extraction component (HPC: Hyper Coal) with high plastic properties to low-quality coals, has been developed.1) On the other hand, many O-functional groups contained in low-quality coals via noncovalent bonds are easily broken during carbonization, and cross-linking, which is the main cause of fluidity decrease, takes place. Thus, it is necessary to suppress cross-linking reactions during carbonization to improve plasticity.2,3) As is well known, noncovalent bonds can easily be severed by highly polar solvents such as pyridine, and the relative amount of noncovalent bonds and the progress of cross-linking during heating can be estimated from the degree of swelling of coal/char.4,5) According to previous work, there is correlation between the carbon content in coal and solvent extraction rate or solvent swelling rate, and the latter two are dependent on the used solvents.6,7,8) In the plot of the carbon content vs. the molecular weight between crosslinks, the maximum value was observed when the carbon content was 86–87 mass%-daf.9,10) Furthermore, the swelling rate and the solubility parameters of solvent, when pyridine extraction residue of Illinois No. 6 coal was immersed in various organic solvents, were investigated. According to this paper, polar solvents such as DMSO, THF and pyridine give higher swelling rates compared with nonpolar solvents such as pentane, benzene or CS2, and pyridine showed the highest value among the tested solvents.7) By applying the coal swelling effect of such solvents, the yield increase of valuable components was attempted by the rapid pyrolysis of a sample in which solvent was introduced into coal.11,12) That is, micropores with the size of the solvent molecules are expanded by solvent treatment, coal was allowed to swell, and solvent and coal were placed close together. Thus, the reaction between solvent-derived radicals and coal fragments was promoted by allowing the pyrolysis rates of coal/solvent to be consistent. Furthermore, it was clarified that noncovalent bonds (hydrogen bonds) in coal are removed by the swelling effect, and cross- linking reaction is suppressed in subsequent pyrolysis.13) It was also reported that the loading rate of coal can be improved by the suppression of cross-linking reactions even at low temperature when pyridine is used.14) Based on such reports, high-strength coke may be produced by removing noncovalent bonds in coal through solvent swelling by filling thermoplastic components, which are considered to be important in coke production, into the coal pores formed by a swelling effect, and by the suppression of cross-linking and close placement of coal-thermoplastic components in the subsequent carbonization process.
The main objective of the present study was to discover a high-strength coke production method. Noncovalent bonds between O-functional groups in coal were cleaved by pyridine, and HPC-derived thermoplastic components were introduced into the pores formed by swelling. Thus, the suppression of cross-linking reactions and the close placement of coal and thermoplastic components were synergistically used to achieve fluidity amplification during carbonization.
Coal samples (GA, TA, KP) with different particle sizes (< 250 μm and 0.5–1.0 mm) and HPC (< 250 μm) provided from the Study Group of Technique Elements for New Cokemaking Process were mainly used. The analysis results for the used coals are shown in Table 1. The C and O contents were 74–88 and 5–19 mass%-daf, respectively. The coal with the particle size of 0.5–1.0 mm was used for coke production.
| Sample | Elemental analysis mass%-daf | Proximate analysis mass%-dry | ||||||
|---|---|---|---|---|---|---|---|---|
| C | H | N | S | Oa | Ash | VMb | FCa,c | |
| KP | 73.6 | 5.5 | 1.5 | 0.7 | 18.7 | 5.8 | 42.9 | 51.3 |
| TA | 83.5 | 5.1 | 2.0 | 0.4 | 9.0 | 6.2 | 37.5 | 56.3 |
| GA | 88.1 | 5.0 | 1.8 | 0.6 | 4.5 | 11.3 | 23.4 | 65.3 |
| HPC | 87.8 | 4.9 | 1.6 | 0.7 | 5.0 | 1.6 | 46.5 | 51.9 |
a Estimated by difference. b Volatile matter. c Fixed carbon.
Chemically modified coal with the HPC-soluble components was prepared by adding 40 mL of pyridine to 1 g of HPC and applying ultrasonic waves for 30 min at room temperature. Subsequently, the mixture was centrifuged at 3500 rpm for 10 min to the separated pyridine-insoluble components (settled solid) and pyridine-soluble components (supernatant) for recovery. Pyridine was added again to the recovered insoluble components, and the above-described extraction was repeated until the pyridine solution became transparent. The recovered insoluble components residue was washed with acetone by applying the above described ultrasonic wave and centrifugation, and then weighed to calculate the extraction rate. The yield of the soluble components was estimated from the pyridine-insoluble components, and it was 15 mass%. Then, TA or KP coal was added into the obtained solution of the pyridine-soluble components, by stirring at room temperature for 24 h, to swell the coal and chemically modify the coal with the soluble components. Subsequently, pyridine was removed at 60°C under reduced pressure, and a chemically modified sample with the HPC pyridine-soluble components was obtained. The loading amount of the soluble components was in the range of 15 to 30 mass% with respect to the amount of coal, and it was controlled by varying the ratio of the solution of the soluble components and the amount of added coal. In addition, pyridine in the solution of the soluble components was removed, without adding coal, under reduced pressure to characterize the pyridine-soluble components, and a solid of the pyridine-soluble components was obtained. For comparison, a similar operation as above was carried out by using methanol (MeOH), which has a swelling effect for coal. The yield of the MeOH-soluble components was 10 mass%, and The loading amount of the MeOH-soluble components was 10 mass%.
We tried to determine the solvent swelling rate for the used coal according to a earlier paper.15) However, the swelling rate could not be determined because of the dissolution of solvent-soluble components in the coal during swelling. Therefore, the swelling rates of the respective coal samples by organic solvents were estimated based on the past report. According to the previous study, in which the relationship between coals with different carbon contents and solvent swelling rates with various solvents was investigated, the swelling rate with methanol of TA coal with 83.5 mass%-daf C was estimated to be 1.05.16) On the other hand, the swelling rate of the pyridine extraction residue of KP coal and that of the coal having the same level of carbon content (79.8–83.1 mass%-daf) as that of TA coal are estimated to be about 2.4 and 2.35, respectively; therefore, coals samples used in this study are considered to have similar swelling rates.7)
2.3. CarbonizationThe production of coke was carried out in a SUS tube.17) A sample with a particle size of 0.5–1.0 mm was charged into the tube (φ 20 mm × height 50 mm), the sample was heated in a muffle furnace, while a load of 200 g was being applied, at 3°C/min up to 1000°C under a stream of N2 and then soaked for 30 min.
2.4. CharacterizationThe functional group analysis for the as–received coals and prepared samples was carried out with a Fourier transform infrared spectrophotometer (FT-IR) (Jasco). A sample mixed with KBr was filled into a press die, and a pellet was prepared by uniaxial pressing with a minipress (Jasco). Subsequently, the sample was analyzed at 5200–400 cm−1 under the conditions of resolution: 0.1 cm−1, accumulation: 200. The obtained spectra were deconvoluted at 1800–1500 cm−1 according to the previous work.18) A thermobalance (Advance Riko) was used for thermogravimetric analysis. A sample was first charged into a Pt pan and then heated to 1000°C at 3°C/min in high-purity He (99.9995%). In order to investigate the thermoplastic properties of the as-received coals and prepared samples during carbonization, photographs were taken at a fixed time interval with a heating microscope (Olympus), while coal particles were heated at 50°C/min. Sample fluidity and contraction behavior during carbonization were investigated with a Gieseler plastometer (Yoshida Seisakusyo) and a high-temperature dilatometer, respectively. The details of those analysis methods have been previously reported.19,20) For the determination of the strength of coke, the maximum load at break was measured with a tensile and compression testing machine (Minebea), and the indirect tensile strength was calculated using the following equation:
Figure 1 shows Gieseler fluidity profiles for GA, TA and KP coals used in this study. TA softened above 380°C, the maximum fluidity (MF) was at about 425°C, and resolidification took place before 460°C. Softening of GA was observed at higher than 410°C, and the fluidity profile gave MF at 460°C. The resolidification temperature was 490°C. On the other hand, KP showed no thermoplasticity. MF values for GA, TA or KP were 2.1, 1.1 or 0 log(ddpm), respectively, and the coal with high O concentration tended to have a lower value.

Gieseler fluidity profile of coals used in this study.
The extraction of HPC using methanol, for which a swelling effect to coal is reported, was carried out. FT-IR of the obtained MeOH-soluble components and that of the insoluble components were measured, and deconvolution was carried out for the observed spectra in the range of 1800–1500 cm−1 (Fig. S1). As a result, absorption bands around 1700 or 1675 cm−1 observed in HPC and assigned to COOH or conjugated C=O became larger in the soluble components. In addition, absorption bands, which have a peak around 1750 cm−1 and were assigned to ester and anhydride, were observed. This peak was not observed in HPC; therefore, the above species that were present in HPC in small amounts are considered to be concentrated in the soluble components when extraction was carried out. On the other hand, O-functional groups (COOH and conjugated C=O) observed in the soluble components were not observed in the insoluble components. These observations indicate that O-functional groups in HPC selectively moved into the soluble components by the MeOH extraction of HPC.
Figure 2 shows the results for the deconvoluted FT-IR spectra of HPC, HPC pyridine-soluble components and HPC pyridine-insoluble components in the range of 1800–1500 cm−1. Absorption bands, assigned to COOH or conjugated C=O and observed at about 1700 or 1675 cm−1 in HPC, disappeared in the pyridine-soluble components and in the pyridine-insoluble components. In addition, the area of the absorption band, observed around 1650 cm−1 in HPC, due to highly conjugated C=O also became smaller in the soluble components and in the insoluble components. The disappearance of absorption peaks, observed around 1700 cm−1 in HPC and assigned to COOH or C=O, may suggest that O-functional groups and N-functional groups are replaced, and that noncovalent bonds between O-functional groups are cleaved during extraction. According to a earlier paper, when lignite is treated with pyridine, pyridine contributes to the cleavage of hydrogen bonds in the coal, and newly N-OH hydrogen bonds are formed.19) It is considered that the interaction between pyridine and O-containing species also takes place in this study; as a result, O-functional groups in HPC probably disappeared or decreased in the soluble components and in the insoluble components. FT-IR spectra of the products obtained by MeOH extraction and pyridine extraction of HPC were very different; therefore, the soluble components in these two solvents are different.

FT-IR spectrum deconvoluted at 1800–1500 cm−1 of HPC, HPC pyridine-soluble components and HPC pyridine-insoluble components.
Figure 3 shows the results for TG analysis of HPC, HPC pyridine-soluble components and HPC pyridine-insoluble components. In the TG analysis of HPC, peaks of weight loss rate were observed at about 250, 380 and 450°C. In the analysis of the pyridine-soluble components itself, a peak was observed at about 100°C; this is due to the release of pyridine adsorbed on the surface of the soluble components. In addition, the peak observed at 380°C with HPC disappeared, and the intensity of the peak at 450°C slightly decreased. On the other hand, peaks around 250°C and 380°C disappeared with the insoluble components, and the peak around 450°C with HPC shifted slightly to a higher temperature. These results indicate that low-molecular components in HPC moved mainly into the soluble components.

Thermogravimetric curves of HPC, HPC pyridine-soluble components and HPC pyridine-insoluble components.
The MeOH-soluble components (Fig. S2) exhibited a drastic weight loss from about 200°C, and a peak of weight loss rate was observed at 300°C. On the other hand, the intensity of the peak, observed at about 250°C with HPC, decreased with the MeOH-insoluble components and the peak around 380°C disappeared; however, the intensity of the peak observed at 450°C with HPC was maintained. That is, low-molecular components in HPC moved into the soluble components by MeOH treatment. The weight loss of the MeOH-soluble components proceeded almost in one step in the range of 200 to 500°C; therefore, low-molecular components are considered to be more abundant in the MeOH-soluble components than in the HPC pyridine-soluble components.
Figure 4 shows TG analysis for the chemically modified sample with the pyridine-soluble components and the physically mixed sample with the pyridine-soluble components. In both cases, the peak around 250°C observed for the soluble components alone almost completely disappeared, the peak intensity at about 450°C also became small, and the decrease was larger with the chemically modified coal. The results suggest that chemical modification promotes the interaction between TA coal and the soluble components more than physical mixing. Similar results were observed for physically mixed and chemically modified coals with the MeOH-soluble components (Fig. S3), and the peak of weight loss behavior observed at about 300°C with the soluble components almost disappeared.

Thermogravimetric curves of TA, HPC pyridine-soluble components, chemically modified and physically mixed samples.
In order to investigate the fluidity of TA samples, the Gieseler test was carried out. In the Gieseler test of chemically modified coal with the pyridine-soluble components by itself, compression did not take place during sample filling. Therefore, measurement with a heating microscope was carried out. Figure 5 shows the results for heating microscope observation of TA and 15 mass% chemically modified TA sample with the pyridine-soluble components. The observation at low heating rate was experimentally difficult; therefore, it was carried out at 50°C/min. The appearance of TA and the chemically modified coal was angular at room temperature. When TA coal was heated, part of particles started to soften at about 450°C, and some swelling was observed at 495°C. In the case of chemically modified coal, on the other hand, part of particles moved in the vicinity of 300°C, particle softening was observed from about 400°C, and the contour shape of softened particles was more rounded at 495°C. The degree of the rounding was also larger than that of TA coal.
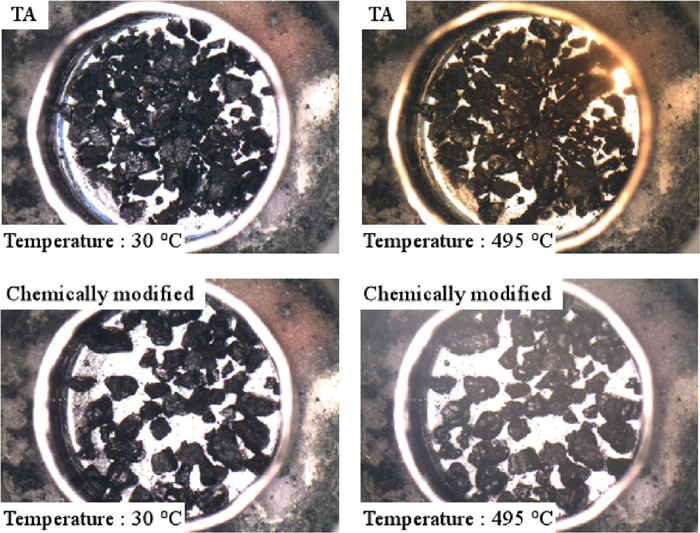
Microscopic pictures of TA and chemically modified sample during heat treatment at 50°C/min. (Online version in color.)
In order to investigate the degree of fluidity improvement when chemical modification with the pyridine-soluble components was done, BW [C, 87; H, 5.0; N, 2.0; S, 0.60; Odiff., 5.8 mass%-daf; ash, 9.6; VM, 57 mass%-dry; MF, 0.30 log(ddpm)], which is slightly-caking coal, and TA sample were mixed in a weight ratio of 7:3, and the fluidity measurement was carried out. Figure 6 shows Gieseler fluidity profiles for the chemically modified sample with the HPC pyridine-soluble components and physically mixed sample with the HPC pyridine-soluble components. In addition, the obtained fluidity properties are provided in Table 2. BW-TA was fluid at 420–460°C and the MF value at about 440°C was 0.60 log(ddpm); the value was very small. Softening of the pyridine-treated coal started from 410°C, the MF value (0.78 log(ddpm)) was observed at 450°C, and then the coal resolidified at 475°C. Some improvement in fluidity was observed for the pyridine-treated coal compared with untreated coal. This may be due to a change in chemical structure, the cleavage of hydrogen bonds caused by the pyridine treatment (reaction of O-functional groups and pyridine) as described above.21) The fluidity property of the physically mixed coal with the soluble components was about the same as that of the pyridine-treated coal; however, the MF value (0.95 log(ddpm)) was slightly larger. On the other hand, the chemically modified coal showed the lowest initial softening temperature (IST) (400°C) among the tested samples, the MF was observed at 450°C and resolidification took place at 470–480°C. The MF value at the loading of 15 mass% was 1.3 log(ddpm); however, the MF value was 1.7 log(ddpm) when the loading was doubled to 30 mass%, and it was more than two times of that for BW-TA. The results are consistent with the results of thermogravimetric analysis in that the soluble components strongly interact with coal in the chemically modified coal. Furthermore, the fluidity of the chemically modified coal drastically improved compared with that of the physically mixed coal; this may be caused by the difference in the contact between coal and the soluble components component.

Gieseler fluidity profiles of BW-TA, pyridine treated, chemically modified and physically mixed samples.
| Sample | Temperature, °C | MFa | ||||
|---|---|---|---|---|---|---|
| ISTb | MFTc | RSTd | FTRe | ddpm | log(ddpm) | |
| 70 mass% BW-30 mass% TA | 420 | 440 | 460 | 40 | 4 | 0.60 |
| Pyridine treated | 410 | 450 | 475 | 65 | 6 | 0.78 |
| Physically mixed (15 mass%-soluble) | 410 | 445 | 475 | 65 | 9 | 0.95 |
| Chemically modified (15 mass%-soluble) | 400 | 450 | 470 | 70 | 19 | 1.3 |
| Chemically modified (30 mass%-soluble) | 400 | 450 | 480 | 80 | 46 | 1.7 |
a Maximum fluidity. b Initial softening temperature. c Maximum fluidity temperature. d Resolidification temperature. e Fluidity temperature range.
On the other hand, the chemically modified TA sample and the physically mixed TA sample with the MeOH-soluble components compressed during sample filling for the Gieseler test; therefore, the prepared samples were not mixed with BW coal. The results for the Gieseler test are shown in Table 3 (Fig. S4). All the samples started to soften at 390–400°C, gave the MF value at 430°C, and resolidified at 450°C. The MF of the chemically modified sample with the MeOH-soluble components and that of the physically mixed sample were 0.90 log(ddpm). Thus, some increase was observed compared with the MF of TA (0.78 log(ddpm)); however, the increase was very small. Thus far, we have investigated Gieseler fluidity when various compounds containing O-functional groups were added to caking coal. We clarified that the fluidity decreases even by the addition of an O-containing compound having a lower boiling point than the IST of caking coal.20,22) In this previous research, it was suggested that O-functional groups may interact with coal particles up to the IST and exert a negative impact on the later fluidity.20) As described above, O-functional groups in HPC are concentrated in the HPC MeOH-soluble components, and the thermal weight loss almost completed before about 400°C where softening of TA starts. It is also evident from the results of TG measurement that the MeOH-soluble components, as well as the pyridine-soluble components, interacts with coal up to the IST of TA. Thus, it is thought that a negative impact to fluidity was exerted by the action of O-functional groups in the MeOH-soluble components, which contains many low-molecular components, to coal up to the IST of TA. That is, it is considered that the TA fluidity hardly changed because of a fluidity improvement effect due to low-molecular components and a negative effect due to O-functional groups.
| Sample | Temperature, °C | MFa | ||||
|---|---|---|---|---|---|---|
| ISTb | MFTc | RSTd | FTRe | ddpm | log(ddpm) | |
| TA | 400 | 430 | 450 | 50 | 4 | 0.78 |
| Physically mixed (10 mass%-soluble) | 395 | 430 | 445 | 50 | 8 | 0.90 |
| Chemically modified (10 mass%-soluble) | 390 | 430 | 450 | 60 | 8 | 0.90 |
a Maximum fluidity. b Initial softening temperature. c Maximum fluidity temperature. d Resolidification temperature. e Fluidity temperature range.
Figure 7 shows the strength of the coke obtained by heating a chemically modified TA sample with pyridine-soluble components (no physical mixing with BW) at 3°C/min to 1000°C. The strength of GA was 3.0 MPa and highly porous areas were observed on the fracture cross section; therefore, the strength may have been reduced by excess swelling. The strengths in the cases of TA, pyridine-treated coal, physically mixed coal with the soluble components and physically mixed coal with HPC were in the range of 1.5 to 3.5 MPa. On the other hand, the chemically modified coal with the soluble components reached 5.2 MPa, it was more than two times of the original coal. However, when the loading was increased by a factor of two, a significant decrease in strength (1.2 MPa) resulted. As described above, the MF increased drastically when the loading of the soluble components was increased; therefore, strength reduction is due to excess swelling. This is supported by the fact that a slight decrease in bulk density was observed when the loading was 30 mass% compared with 15 mass% loading. The yield and bulk density became smaller, in the case of the chemically modified coal with the soluble components, than TA and physically mixed coal; this is considered to be due to the release of pyridine, which adhered to the sample during sample preparation. On the other hand, the yield of coke prepared from 15 mass% chemically modified sample and that of coke from 30 mass% chemically modified sample were comparable. This strongly indicates that the soluble components component interacts with coal during carbonization process and is incorporated into the solid phase. When HPC was physically mixed, the strength was 3.5 MPa; this value is roughly the same as the value for the physically mixed coal with the soluble components. From the thermogravimetric analysis results of the HPC pyridine extracts, the molecular weight of the pyridine-soluble components is lower compared with that of the pyridine-insoluble components. This may thus be suggesting that relatively low-molecular weight components in HPC contribute to the increase of coke strength when HPC is added to TA. On the other hand, the coke strength from the chemically modified coal was larger than that from the physically mixed coal; this is considered to be due to a difference in the initial dispersion of the soluble components into coal. That is, the contact of coal-the soluble components and their interaction during carbonization were improved by loading the soluble components inside the pores formed by coal swelling in pyridine and the fluidity was increased; as a result, the coke strength presumably increased. As is well known, asphalt pitch (ASP) is used as a caking additive for the production of high-strength coke. The mechanism of coke quality improvement, by the addition of ASP, is understood in that the supply and miscibility of caking component were improved, because of high fluidity, and the optical anisotropy was developed in the coke structure.23) It is also reported that, in the coke produced from ASP-added coal, the pore shape and pore wall thickness change, and the rate of fine foaming decreases; as a result, the coke strength increases.24) In addition, the strength of produced coke improves by finely divided ASP.24) That is, the dispersion of caking additive is one of the factors that affect the strength of coke. In the chemically modified coal with the HPC pyridine-soluble components, thermoplastic components dissolved in pyridine are impregnated into the pores generated by the swelling effect of pyridine, and the soluble components and coal are located close. Thus, the action similar to that of ASP (change in the pore shape and pore wall thickness due to fluidity improvement) may have improved fluidity and the coke strength. Thus, it was found that the strength of coke could be improved by the present method in which TA coal is chemically modified with HPC pyridine-soluble components.

Properties of cokes and products prepared from TA samples.
In order to understand the improvement of coke strength of the chemically modified coal with the pyridine-soluble components, the fracture cross section was polished after coke crushing test; SEM observation was carried out, and the results are shown in Fig. 8. In TA coal, partially softened structure was observed; however, the interface contact between the reactive component and inert component was small, as shown in the enlarged picture. On the other hand, in the coke prepared from the chemically modified sample with the soluble components (15 mass%), numerous softened structures were observed. Furthermore, the interface contact between the softened section and inert component was good. Thus, the thermoplastic property during carbonization was improved by chemical modification with the soluble components; as a result, the contact between the reactive component and inert segment improved and the coke strength increased.

SEM pictures of cross-section of TA and chemically modified TA sample with HPC pyridine-soluble components.
The strength of coke obtained by heating the chemically modified KP sample with the pyridine-soluble components (no physical mixing with BW) at 3°C/min to 1000°C is shown in Fig. 9. The products obtained from KP, pyridine-treated coal and chemically modified coal with the soluble components (15 mass%) were not coked, and the yield was in the range of 45 to 55 mass%-dry. On the other hand, in the case of 30 mass% chemically modified coal with the soluble components, coke could be produced, and the strength was 0.5 MPa. The coke strength of the physically mixed coal with the soluble components and that of the physically mixed coal with HPC were 2.0–3.2 MPa, and unlike TA, a high strength was observed with physically mixed coal. The reason for the larger coke strength by physical mixing than chemical modification is unknown at present. For coals such as KP having no fluidity, however, the use of additives such as HPC containing both low-molecular and high-molecular components (component that is fluid at low temperature and component that is fluid at high temperature) is suggested to be important for the improvement of coke strength.

Properties of cokes and products prepared from KP samples.
Figure 10 shows the results for the contraction behavior of TA samples. The behavior above 450°C after the release of volatile matters from TA and the chemically modified sample with the 15 mass%-soluble components were about the same. Similar results were also observed for pyridine-treated coal and the physically mixed coal with the soluble components. Figure 11 summarizes the contraction rate of each sample determined from the corresponding contraction behavior shown in Fig. 10. For comparison, the contraction rate for typical caking coal and that for non- or slightly-caking coal were also provided. The contraction rate of TA coal was about 13.5%, and the pyridine-treated coal, chemically modified coal with the soluble components, physically mixed coal with the soluble components and HPC physically mixed coal had almost the same contraction rate. Thus, it was found that the present method hardly affects the rate of contraction.

Contraction profiles of TA and chemically modified TA sample with HPC pyridine-soluble components.

Summary of contraction rate of TA samples.
In this study, noncovalent bonds between O-functional groups in coal were cleaved by pyridine containing HPC pyridine soluble, and HPC-derived thermoplastic components were introduced into the pores formed by swelling; thus, the synergistic effect during carbonization of the suppression of cross-linking reactions and the fluidity amplification due to close placement of coal and thermoplastic components was utilized. In the investigation of a high-strength coke production method, the following conclusions were obtained.
(1) When HPC was extracted with pyridine, a decrease in O-functional groups was observed in the obtained pyridine-soluble components and pyridine-insoluble components. Thus, it is considered to be possible to decrease the extent of hydrogen bonding between O-functional groups in HPC by pyridine extraction. On the other hand, O-functional groups in HPC selectively moved into the soluble components by MeOH extraction.
(2) When non- or slightly-caking coal was chemically modified with the HPC pyridine-soluble components by utilizing a solvent swelling effect of pyridine, fluidity increased compared with the coals physically mixed with the soluble components or HPC. On the other hand, the fluidity of the chemically modified sample with the MeOH-soluble components hardly changed from that of the original sample, and no effect of the modification with the thermoplastic component was observed.
(3) It was clarified that higher strength coke can be produced from the chemically modified sample with the HPC pyridine-soluble components than from the original coal or the physically mixed coal with the soluble components.
(4) A large difference was not observed during carbonization in the contraction behavior of the chemically modified sample with the soluble components and that of the original coal.