2019 Volume 59 Issue 8 Pages 1419-1426
2019 Volume 59 Issue 8 Pages 1419-1426
In the present study, we prepare several types of specimens from non-caking coal — including specimens in which noncovalent bonds between O-functional groups in coal are cleaved by pyridine and HPC-derived thermoplastic components are introduced into the pores produced by swelling, as well as specimens consisting of physical blends with HPC — and examine the influence of heating conditions and types of caking agents on the production of high-strength coke using a SUS tube. We also investigate the influence of heating conditions and types of caking agents on the strength of coke from pelleted specimens and determine the optimal conditions for producing high-strength coke from non-caking coal. HPC with a wide range of thermoplastic properties is more effective as caking agents than additives containing only low- molecular-weight or high-molecular-weight components. In addition, the strength of the produced coke depends on the amount of the additive, and optimal values of the additive amount are present. It was found that the following heating schedule is effective for producing high-strength coke from non-caking coal with added caking agents: First, high-speed heating (20°C/min) to an intermediate temperature in the range 400–600°C, recognized as the thermoplastic temperature range for typical caking coal; then, low-speed heating (3°C/min) to the temperature range of 900–1000°C. Moreover, we demonstrate that, by increasing the rate of heating in the thermoplastic temperature range, it is possible to reduce the amount of caking agent added.
The development of methods for producing coke from low-quality coal (non-caking or slightly-caking coal), which is inexpensive and plentiful, is important for expanding the range of resources from which coke can be produced. Because low-quality coals typically fail to become thermoplastic during carbonization, the production of coke from low-quality input coals generally requires control of the heating conditions1,2,3) and the addition of caking agents4,5) to lend fluidity. In recent years, Hyper Coal (HPC), which exhibits extremely high fluidity as a caking agent, has become a focus of interest, with the high thermoplasticity and permeability exhibited by HPC upon heating serving to increase the fluidity in blended coal systems. Moreover, because HPC penetrates into the gaps between surrounding blended-coal particles and fuses — even at relatively low temperatures — it fills the spaces between particles, adding cohesion and increasing fused components.5) On the other hand, low-quality coal contains large amounts of O-functional groups, and reactions that occur in the carbonization process create interlinkages between the oxygen atoms of these functional groups, a phenomenon that is believed to obstruct thermoplastic properties.6,7) In previous work, we have investigated methods for exploiting the specific effects of pyridine on coal – namely, solvent swelling and the cleavage of noncovalent bonds — to suppress cross-linking reactions during carbonization and amplify thermoplastic components, facilitating the development of methods for producing high-strength coke from non-/slightly-caking coal. In particular, we demonstrated that coke manufactured from a specially-prepared input material — in which HPC pyridine-soluble components are chemically-loaded into the pores produced by pyridine treatment of coal — is stronger than that produced from the unloaded input material.8,9) We attributed this result to a synergistic interplay of two mechanisms: (a) pyridine-induced cleavage of noncovalent bonds between O-functional groups in coal, and (b) suppression of cross-linking reactions during carbonization and amplification of fluidity due to increased proximity between coal and thermoplastic components, resulting from the introduction of HPC-derived thermoplastic components into the pores produced by pyridine-induced swelling.8,9) In addition, we showed that the extent of the improvement in strength is greater for formed coke.9)
In the present study, we first apply the above methods to non-caking coal. We prepare two specimens from non-caking coal — one in which noncovalent bonds between O-functional groups in coal have been cleaved by pyridine and HPC-derived thermoplastic components have been introduced into the pores produced by swelling, and a second specimen consisting of a physical blend with HPC — and investigate the influence of heating conditions and types of caking agents on the production of high-strength coke using a SUS tube. We also examine the influence of heating conditions and types of caking agents on the strength of coke prepared via forming methods and finally determine the optimal conditions for producing high-strength coke from non-caking coal.
Coal samples (GA, KP) of varying particle sizes (< 250 μm and 0.5–1.0 mm) and HPC (< 250 μm) provided from the Study Group of Technique Elements for New Cokemaking Process8,9) were used. The C and O contents of the coals used were 74–88 and 5–19 mass%-daf, respectively.8,9) The composition of HPC was: 88% (mass%-daf) C, 4.9% H, 1.6% N, 0.7% S and 5.0% O.8,9) For GA, the initial softening temperature (IST) was 410°C, and a maximum fluidity (MF) of 130 ddpm [2.1 log(ddpm)] was observed near 460°C, before resolidification at 490°C. The fluidity temperature range was 80°C. KP did not exhibit a transition to fluidity.8,9)
2.2. Preparation of Coal with Chemically-Loaded HPC Pyridine-Soluble ComponentsPreparation of coal containing chemically-loaded HPC pyridine-soluble components followed previous reports. The details of our procedure were as reported in Refs. 8 and 9. After HPC was extracted by pyridine at ambient temperature, and then coal was added to a solution of pyridine-soluble HPC. The mixture was stirred at room temperature for 24 h to achieve swelling and loading of the pyridine-soluble components. Finally, the pyridine in the mixture was eliminated via pressure-reduced heating to prepare samples containing chemically-loaded pyridine-soluble HPC. The amount of loaded soluble components lies in the range 0–50 mass% with respect to coal. Here, the remained pyridine in solid of HPC pyridine soluble was approximately 15 mass%.8) In addition, The yield of the soluble components was estimated from the pyridine-insoluble components obtained after acetone washing, and it was 15 mass%.8)
2.3. CarbonizationCarbonization was carried out in a SUS tube to produce coke.8,9,10) After charging the tube with specimens of particle size 0.5–1.0 mm, the sample was carbonized for 30 min in a muffle furnace under N2 gas flow at 1000°C. For coke preparation in the tube, several different heating schedules (Cases 1–4) were considered. In Case 1, the sample was heated at a constant rate of 3°C/min up to a temperature of 1000°C. In Cases 2–4, the sample first was heated at 20°C/min to 400, 500 and 600°C, respectively, and then was continued heating at 3°C/min to 1000°C.9) To prepare formed coke, coal of particle size < 250 μm was used, as well as our prepared specimens. After pelletizing specimens at 30 MPa, the samples was carbonized for 30 min at 900°C under heating patterns similar to the above.9) For preparation of formed coke, 4 distinct heating schedules (Cases 5–8) were considered. In Case 5, the sample was heated at a constant rate of 3°C/min up to a temperature of 900°C. In Cases 6–8, the sample first was heat at 20°C/min to 400, 500 and 600°C, respectively, and then was continued heating at 3°C/min to 900°C. In this study, the coke prepared at heating rates of 3 and 20°C/min respectively were denoted as low-speed heating and high-speed heating. The coke prepared from pelleted coal was denoted as formed coke.
The strength of prepared coke samples was characterized destructively by measuring maximum loads, and then calculating indirect tensile strength using the following equation:8,9)
Figure 1 shows the strength of coke prepared from GA and KP specimens under various heating conditions. For Case 1, in which we heat at 3°C/min to 1000°C (Fig. 1(a)), the strength of the coke prepared from GA is 3.2 MPa. For KP in Case 1, we were unable to make strength measurements, as none of the KP samples produced coke with this heating schedule. For Case 2, in which the sample was heated at 20°C/min to 400°C and then was continued carbonizing at 3°C/min to 1000°C (Fig. 1(b)), the strength of the coke from GA decreased to 1.5 MPa. In this case, all KP specimens produced coke. For coke produced from the 4 KP specimens — untreated KP, KP with a physical blend of 15 mass% HPC, and KP with 15 and 30 mass% chemically-loaded pyridine-soluble HPC — the strengths were 0.1, 3.5, 1.5, and 2.0 MPa, respectively. The order of KP specimens according to the strength of coke produced was untreated < 15 mass% chemically-loaded < 30 mass% chemically-loaded < HPC physical blend. Similar strength increases were observed for coke produced from KP specimens at the heating schedules of Cases 3 and 4, which involve high-speed heating at 20°C/min to 500–600°C (Figs. 1(c) and 1(d)).

Effect of heating conditions on coke strength of GA and KP samples: (a) Case 1, (b) Case 2, (c) Case 3, and (d) Case 4.
Figure 2 shows the influence of heating conditions on the bulk density and yield of prepared coke (Fig. 2(a)) and the relationship between the strength (Fig. 1) and bulk density or yield (Fig. 2(b)). Although the data are somewhat scattered, the bulk densities and yields of the coke prepared from the various specimens remained roughly constant independent of the heating conditions (Fig. 2(a)). The bulk density tends to increase as the strength increases; however, no clear correlation is observed between the yield and strength (Fig. 2(b)). These results indicate that the addition of caking agent to KP — a non-caking coal — creates fluidity during carbonization, increasing the fine-grained density of interaction between carbonaceous materials. The relation between KP-produced coke strength and heating conditions shown in Fig. 1 demonstrates that, by varying heating conditions, it is possible to induce coke formation and increase strength even with non-caking coal as input material. This shows that even non-caking coal contains fluid components. In previous work, we have used thermogravimetric analysis to show that the soluble and insoluble components of HPC obtained via separation by pyridine solvation consist, respectively, of the low-molecular-weight and high-molecular-weight components of HPC.8) In this study, the increased strength of coke prepared from specimens with chemically-loaded HPC pyridine-soluble components may be attributed to the contribution of low-molecular-weight components in HPC (together with the cleavage of interlinkages). On the other hand, HPC is a continuous fluid material composed of low- and high-molecular-weight components. A comparison of coke strength in the two cases shows that the extent of the contribution of the respective components to coke strength is greater for the continuous fluid material as HPC than for the low-molecular-weight components alone. This point is discussed in greater detail below (section 3.3). Our findings demonstrate that, when using a SUS tube to prepare high-strength coke from non-caking coals such as KP, an effective heating strategy is to heat at high speed (20°C/min) through the thermoplastic temperature range of the input coal to temperatures of 400–600°C, and then switch to low-speed heating (3°C/min) and continue heating to 1000°C, as in our Cases 2–4.

Change in bulk density and coke yield under different heating conditions (a) and relationship between indirect tensile strength and bulk density or coke yield (b).
Figure 3 compares the indirect tensile strength of formed coke prepared from KP specimens under various heating conditions. Figure 4 shows the results of Fig. 3 arranged by heating condition. The strength of coke prepared from GA under the heating conditions of Cases 5 and 6 was above 13 MPa in all cases, while for Cases 7 and 8 the strengths were less than 1.0 MPa (Fig. 4(a)). Figure 5 shows the bulk density (Fig. 5(a)) and yield (Fig. 5(b)) for prepared cokes corresponding to Fig. 3. From the dramatic decrease in density observed for GA in Cases 7 and 8, it was concluded that the observed drop in strength is due to the formation of foam-like structures due to excessive swelling. For coke prepared from untreated KP and pyridine-treated KP, the strength, yield and bulk density remained roughly constant independent of the heating conditions, although the data are somewhat scattered (Figs. 4(b), 5(a) and 5(b)). Previous studies of the production of formed coke have reported that plasticity is changed by factors such as immersion of coal in organic solvents or adsorption.11,12,13) Although the extent of this effect depends on factors such as the particular organic solvent and coal rank, the use of pyridine with coal of carbon content corresponding to non-/slightly-caking coal (80 mass%-daf) causes a major reduction in Knoop hardness.14) Consequently, pyridine treatment is thought to increase formability and plasticity by increasing contact area between coal particles, producing coke of greater strength.14) For a physical blend of HPC pyridine-soluble components (additive amount, 15 mass%), a strength of 13 MPa — comparable to that for GA — is attained in Cases 5 and 6. However, the strength decreases in Cases 7 and 8, in which the high-speed (20°C/min) heating extends to higher temperatures (Fig. 4(d)). Similar behavior is observed for specimens with additive amount of 30 mass%, but with strengths lower than those for the corresponding 15 mass% specimens; the bulk density is also higher for the 15 mass% specimens. On the other hand, for the chemically-loaded specimens (for both additive amounts 15 mass% and 30 mass%), although the data are somewhat scattered, coke strength decreased as the heating rate increased. Also, the strength was greater for greater amounts of loaded additive. In contrast, the bulk density remained roughly constant independent of heating condition (Fig. 5(a)). Although previous work of slightly-caking coal have reported that the strength of formed coke prepared from chemically-loaded specimens is greater than for specimens with physically-blended HPC soluble components,9) here we find the opposite for non-caking coal. Although the reason for it is unclear at present, the increasing strength of formed coke accompanying increased amounts of loaded pyridine-soluble components, discussed below, indicates that, whereas the soluble components serve as a connecting agent between particles (particle-particle interfaces) in physical blends, for chemically-loaded coals the introduction of mobile species from soluble components into pores tends to consume mobile species in stabilization as non-mobile matter (the amount of soluble component present on particle surfaces is less than in the physical-blend case), which may result in lower strength. In the future, it may be necessary to investigate in detail such factors as the state of loaded soluble components and their dispersiveness. Figure 6 shows the result of an investigation of the relationship between coke strength and bulk density or yield corresponding to Figs. 3, 4 and 5. The bulk density increases as the coke strength increases, whereas the yield decreases as the coke strength increases. However, if we look only at systems to which caking agents were added, the yield is roughly constant (slightly reduced compared to KP). This indicates that, while a portion of the caking agent vaporizes during carbonization, the majority is retained in KP.

Indirect tensile strength of formed coke prepared from KP samples for Case 5 (a), Case 6 (b), Case 7 (c) and Case 8 (d).

Indirect tensile strength of formed coke prepared from GA (a), KP (b), pyridine treated (c), physical mixture (15 mass%) (d), physical mixture (30 mass%) (e), chemically-loaded (15 mass%) (f) and chemically-loaded (30 mass%) (g).

Changes in bulk density (a) and coke yield (b) of formed coke prepared from KP sample under different heating conditions.

Relationship between indirect tensile strength and bulk density or coke yield corresponding with the result of Fig. 4.
To study the influence of types of caking agents, the amount of caking-agent additives and the heating conditions on the strength of formed coke, we investigated the properties of coke as the types and amount of additive as well as the heating conditions were varied. Figure 7(a) shows the influence of heating conditions on the strength of coke prepared from a physical blend of HPC with KP. The KP used for this test was taken from lots different from that used in Fig. 3. Although the elemental analysis was carried out for different lot sample, the values were almost same with the values in Section 2.1. For Case 5, the strength of coke initially increases as the amount of HPC additive increases, peaking at a maximum strength of 9.0 MPa at additive amount 60 mass%, and thereafter falling to 3.0 MPa at 70 mass%. On the other hand, for Case 6 the maximum strength is 10 MPa at 50 mass%, falling to 4.0 MPa at 60 mass%. For Cases 7 and 8, the trends of strength vs. additive amount were roughly similar, peaking at a maximum strength of 10 MPa at 40 mass% and thereafter decreasing in strength as the additive amount increases. From the variation of bulk density vs. additive amount shown in Fig. 7(b), it was concluded that the decrease in strength for increasing additive amount is due to an excess of foam-like structures due to increased swelling. The yield remained essentially constant independent of additive amount. Figure 8 plots the relationships between coke strength and yield, and between coke strength and bulk density, corresponding to Fig. 7. Data points indicating low density arise from the formation of foam-like structures due to excessive swelling; disregarding these points, the density and yield remained essentially constant independent of strength. These results indicate that HPC interacts with KP (Fig. 7(b)) and is retained in the solid phase. In addition, it have been found that, by increasing the rate of temperature increase and the temperature range subjected to high-speed heating, it is possible to produce high-strength coke from non-caking coal even with reduced amounts of HPC additive.

Changes in indirect tensile strength (a), bulk density and coke yield (b) of coke prepared under different heating conditions against physically mixed amount of HPC to KP.

Relationship between indirect tensile strength and bulk density or cake yield corresponding with the result of Fig. 7.
Figure 9 shows the strength (Fig. 9(a)), the bulk density and yield (Fig. 9(b)) of coke prepared under various heating conditions from KP taken from the same lots as in Fig. 7 with varying amounts of chemically-loaded HPC pyridine-soluble components. In the low heating rate carbonization of Case 5, the coke strength is 5.0 MPa at an additive amount of 50 mass% (Fig. 9(a)), while for Case 6 with the same additive amount the strength increases to 9.0 MPa. On the other hand, for the cases involving high-speed heating to 500–600°C (Cases 7–8), the strengths of the cokes prepared at additive amount 50 mass% were 10.5 and 11 MPa respectively, indicating that the maximal strengths (among the additive amounts tested) were obtained for smaller additive amounts than in the previous two cases. This trend in strength vs. additive amount differs from that of Fig. 7 for HPC additive; also, no maximum strength was observed in the range tested, suggesting that higher additive amounts are required compared to the HPC case. The variation in bulk density with additive amount shown in Fig. 9(b) increases slightly at an additive amount of 50 mass%, while the yield remains roughly constant independent of additive amount. Thermogravimetric analysis of the HPC pyridine-soluble components indicates reduced mass for the soluble components alone starting in the low-temperature region;8) for this reason, we expected the yield to decrease as the amount of loaded additive increased, but no such trend was observed. Here, carbonization yields of the coal and HPC pyridine soluble are approximately 60 and 30 mass%, respectively. The calculated carbonization yields based on these yield at various mixture ratio of coal and the soluble ranged at 30–46 mass%-dry. These calculation values differed from those of measurement values of yield (approximately 50 mass%). Figure 10 shows the relationships between coke strength and bulk density and between coke strength and yield. The density increases with increasing strength, indicating that the increased fluidity resulting from the chemically-loaded soluble component yields a more fine-grained structure in the coke produced. The relationship between strength and bulk density in Fig. 10 gave different tendency with Fig. 8. This difference may occur due to distinction of mobile form, (HPC (light and heavy component) in Fig. 8 and HPC pyridine soluble (light component) in Fig. 10). In addition, blending method may give different effect. In other word, the effect of chemically modified loading on the strength and bulk density is greater than that of HPC physical mixture. On the other hand, the yield is roughly independent of strength, which indicates that the chemically-loaded soluble components interacts with KP in the carbonization process.
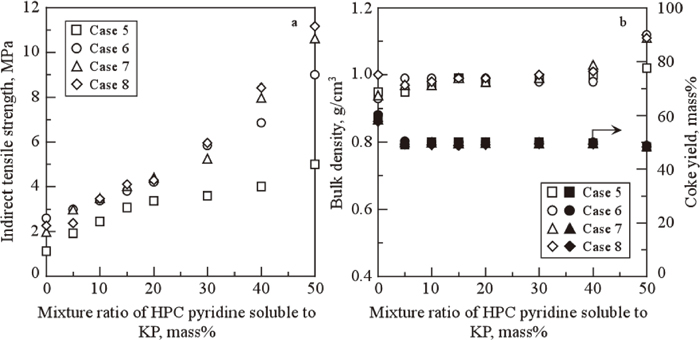
Changes in indirect tensile strength (a), bulk density and coke yield (b) of coke prepared under different heating conditions against chemically-loaded amount of HPC pyridine-soluble components to KP.

Relationship between indirect tensile strength and bulk density or coke yield corresponding with the result of Fig. 9.
Figure 11 shows the strength, bulk density and yield of coke prepared from KP with physical blends of varying amounts of HPC pyridine-insoluble components under various heating conditions. The KP used for this test was taken from the same lots used in Fig. 9. For Case 5, the coke strength at a 50 mass% blend was only 2.5 MPa, a small value. In contrast, for Case 6 the strength increased to 5.0 MPa at 50 mass%, and in cases 7 and 8 the strength attained a maximum value of 7.0 MPa at 30 mass%. For all heating conditions, no decrease in strength was observed to accompany increasing amounts of additive. In other words, further strength increases may be possible for further increases in additive amount. Although this trend of increasing strength is similar to that observed for chemically-loaded HPC pyridine-soluble (Fig. 9(a)), the extent of the increase is lower. Figures 11(b) and 12 show the variation in bulk density and yield accompanying changes in coke strength and the amount of the insoluble component additive corresponding to Fig. 11(a). As for the case of the chemically-loaded soluble components, the yield remains roughly constant independent of blend amount and coke strength, while the bulk density increases. Thus, even for insoluble component additives, the insoluble components interact with KP during carbonization, resulting in fluidity that promotes the densification of the base coke substance.

Changes in indirect tensile strength (a), bulk density and coke yield (b) of coke prepared under different heating conditions against phys-ically mixed amount of HPC pyridine-insoluble components to KP.

Relationship between indirect tensile strength and bulk density or coke yield corresponding with the result of Fig. 11.
A comparison of the results from physically-blended HPC (Fig. 7), chemically-loaded pyridine-soluble HPC (Fig. 9) and physically-blended pyridine-insoluble HPC (Fig. 10) shows that the strength is lower in the latter two cases. As mentioned above, the pyridine-soluble and insoluble components of HPC may be thought of as the low- and high-molecular-weight constituents of HPC, separated by solvation.8) Therefore, the pyridine-soluble components correspond to low-molecular-weight mobile components, while the pyridine-insoluble components correspond to high-molecular-weight mobile components. A comparison of the strength of coke and formed coke prepared from KP under various heating conditions shown in Figs. 1 and 4(b) indicates that the strengths of the various cokes increase as the heating rate or temperature range of rapid heating rate are increased; as noted above, this indicates that, even for non-caking coal, it is possible to achieve fluidic behavior by increasing the heating rate or temperature. In the continuous self-dissolution model, the mobile components produced at the early stages of carbonization dissolve the higher-mass immobile components, yielding continuous fluidic behavior.14) In this work, we expected that the increase in fluidic components at the early stages of carbonization due to chemically-loaded HPC pyridine-soluble components would increase the intrinsic fluidic behavior of KP, stimulating the dissolution of immobile components at later stages, increasing fluidity, and thus increasing coke strength; however, in practice the strength was less than that observed for HPC additives. This may be due to a relative paucity of immobile components, which are dissolved by mobile components contained in KP that are produced at the early stages. Meanwhile, the addition of the pyridine-insoluble components — which are thought to contain more high-molecular-weight components than the pyridine-soluble components — resulted in coke strength inferior to that of both the HPC-additive and chemically-loaded pyridine-soluble cases. These findings indicate that the influence of low-molecular-weight components on coke strength is greater than the influence of high-molecular-weight components; however, observations of increased coke strength show that the presence of high-molecular-weight components in HPC is also important for increasing coke strength. Moreover, the finding that HPC additives can provide greater increases in strength than low-molecular-weight or high-molecular-weight additives alone indicates that the addition of caking agents consisting of continuous bodies of low- and high-molecular-weight components is important for increasing the strength of coke produced from non-caking coal. These results demonstrate that, in producing high-strength coke from non-caking coal, it is important to add caking agents that exhibit fluidity over a wide range, and to raise the temperature rapidly through the fluidity temperature range (400–600°C) recognized for strongly-caking and slightly-caking coals.
(1) When producing high-strength coke from non-caking coal using a SUS tube or pelletization method, the addition of HPC — which exhibits a wide range of thermoplasticity — is more effective than the addition of low- or high-molecular weight fluid components alone as a caking agent.
(2) In addition, the strength of coke produced depends on the amount of added caking agents, and an optimal additive amount exists.
(3) When preparing high-strength coke from non-caking coals with added caking agents, an effective heating strategy is to heat at high speed (20°C/min) to temperatures of 400–600°C — the thermoplastic temperature range generally observed for caking coals — and then switch to low-speed heating (3°C/min) and continue heating to 900–1000°C.
(4) The amount of caking agent added may be reduced by increasing the heating rate in the thermoplastic temperature region.