2020 Volume 60 Issue 11 Pages 2519-2524
2020 Volume 60 Issue 11 Pages 2519-2524
It is well known that Si, Mn and B, the alloying elements for high-strength steel sheets, easily form oxides on the steel surface during annealing in a reducing atmosphere, and those oxides have a large influence on the surface performance of steel sheets, such as phosphatability. In this work, we discovered that the oxidation behavior of Mn-added high strength cold-rolled steel sheets could be simulated on mild steel sheets by using an ion plating method and investigated the relationship between the morphology of Mn oxides and phosphatability under the condition that both the amount and kind of Mn oxides were fixed. In a simulated Mn–O layer, fine surface oxides, which covering most of the steel surface, were observed after annealing. On the other hand, in a Mn–B–O layer, large globular surface oxides were observed on the steel surface, and the Fe surface was partially bare. The B–Mn compound oxide is considered to be in a molten phase during annealing because the melting point of the compound oxide is lower than the annealing temperature, and as a result, it is thought that large B–Mn compound oxides coagulate and grow during annealing. In addition, it was found that the large B–Mn compound oxides (about 500 nm) interfere with steel dissolution in the phosphate solution. These results demonstrate the importance of controlling the morphology as well as the amount and kind of surface oxides for obtaining good phosphatability of Mn-added high strength cold-rolled steel sheets.
Steel sheets used in automobile bodies should have high strength from the viewpoint of weight reduction accompanying the tightening of exhaust gas regulations while ensuring the safety of the occupants. Because high-strength steel sheets must be press-formed into complex shapes depending on the automobile part, sufficiently high formability capable of withstanding forming into such complex shapes is also required for practical application.1,2,3)
Manufacturing of high-strength cold-rolled steel sheets includes an annealing process at high temperatures ranging from 750°C to 900°C, during which the microstructure of the steel sheet is recovered by eliminating strain introduced by the cold-rolling process.
Furthermore, to ensure adequate strength and formability after the annealing process, various elements are added to the steel sheet in advance.4,5,6) Although the heating atmosphere during the annealing process for high-strength cold-rolled steel sheets is thermodynamically reducing for iron, it is oxidizing for silicon (Si), manganese (Mn), and boron (B), which are referred to as easily oxidizable elements.7,8,9,10,11,12) During annealing, these elements diffuse from the interior of the steel sheet to the sheet surface, where they form surface oxides by precipitating and concentrating at concentration levels higher than those inside the sheet via selective surface oxidation reactions. However, these oxidation processes are very complex because the amount, kind, and morphology (size and shape) of the oxides are strongly influenced by the atmosphere (kind, concentration, and dew point of the gas) during annealing and by the heating conditions (temperature and time).10,11,12)
Surface oxides formed on a steel sheet are known to adversely affect the surface preparation for painting,13,14,15) i.e., phosphating, which is used to improve the adhesion between the steel sheet and the paint film.16,17,18) Therefore, to obtain good phosphatability, it is important to dissolve the oxides on the steel sheet surface in the phosphating solution. The dissolution speed of oxides, which is specific to the amount and kind of oxides, and the oxide size, are considered to influence their dissolution. However, as previously described, it is impossible to independently control the amount, kind, and morphology (size and shape) of the oxides formed on the surface of a high-strength cold-rolled steel sheet during annealing. Consequently, previous studies have investigated only the effects of the kind and morphology of the oxides on phosphatability under different oxide amounts.17,18) To the best of our knowledge, no published report to date has investigated the effect of the oxide morphology on phosphatability under a condition in which the amount and kind of the oxides were controlled.
Therefore, we propose a new method for creating a surface simulating that of a high-strength cold-rolled steel sheet, wherein the amount and kind of oxides are independently controlled, on a mild steel surface by adding oxides to the steel sheet surface by an ion-plating method, followed by annealing. Focusing on Mn and B, which are generally added to high-strength cold-rolled steel sheets as easily oxidizable elements, we report the relationship between the morphology of manganese oxides and phosphatability when the amount and kind of manganese oxides are controlled.
The sample materials used here were prepared by the following procedure. First, the composition of a steel sheet was adjusted in the laboratory to 0.001 mass% C, 0.01 mass% Si, and 0.12 mass% Mn, corresponding to the composition of the cold-rolled mild steel generally used for automobile steel sheets. Next, 50 kg of steel was melted under a vacuum and cold-rolled after the hot-rolling process. The thickness of the steel sheet samples was 0.8 mm. After the cold-rolling process, the following pretreatments were conducted: 1) electrolytic degreasing in a 3 mass% NaOH solution at 60°C for 30 s at a current density of 790 A/m2 and 2) immersion treatment in 5 mass% HCl at 60°C for 6 s. Then, the surface oxide films, i.e., Mn–O and Mn–B–O films shown in Fig. 1, were formed on the steel sheet by using a high-frequency excitation instrument (SHOWA SHINKU Co., Ltd.) using MnO2 and B2O3 (ADVANTEC Co., Ltd.) as evaporation target materials. According to previous reports,9,10) ultra-trace amounts of B tend to change the size of manganese oxides without changing the manganese oxide type. Therefore, we also prepared a Mn–B–O film in order to prepare a sample with substantially a different manganese oxide morphology. The thickness of the target oxide film was 50 nm, which is similar to the thickness of the oxide film formed on real high-strength steel sheets.19,20) Next, the sample was heated to 900°C in a 5 vol% H2-95 vol% N2 atmosphere at a rate of 10°C/s. Annealing was then conducted by maintaining the sample at 900°C for 120 s. After the annealing process, the sample was cooled to room temperature under nitrogen gas.
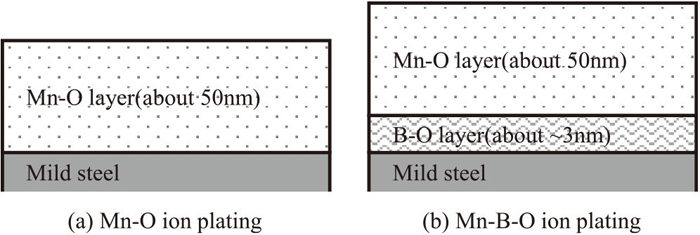
Cross-sectional diagrams of mild steel after ion plating.
To investigate the oxide film formed on the surface of the steel sheet by ion plating, an observation test specimen with a 45° cross section was prepared by using a focused ion beam. The oxide film was observed by scanning electron microscopy (SEM) on an electron microscope operating at an acceleration voltage of 5 kV, and the depth profile of the element concentration was measured by glow discharge optical emission spectrometry (GD-OES). The sputtering rate shown on the horizontal axis was 3.5 nm/s when converted to that of MnO. Next, the oxide formed during annealing was identified by thin-film X-ray diffraction (XRD) using Cu-Kα X-rays generated at a voltage of 55 kV and current of 250 mA; the X-ray incident angle was 2°. In addition, the surface oxides were observed by SEM at an accelerating voltage of 1 kV. To analyze the composition and distribution of the materials confirmed by the surface observation, qualitative analysis was conducted by energy-dispersive X-ray spectrometry (EDS).
2.3. Evaluation of PhosphatabilityPhosphating of the sample materials was conducted by using a commercially-available phosphating solution conventionally utilized for the surface treatment of automobiles (Nippon Paint Surf Chemicals Co., Ltd., Surfdine EC1000). The surface coating state of the phosphate crystals on the steel sheet was observed by SEM at an accelerating voltage of 15 kV, and the amount of adhering phosphate crystals was measured by X-ray fluorescence analysis.
Figures 2(a) and 2(b) show SEM images of the cross sections of the Mn–O and Mn–B–O films produced by ion plating. In both images, ion-plating films with thicknesses in the order of tens of nanometers were observed on the surface of the steel sheet. Figures 3(a) and 3(b) show the concentration profiles measured by GD-OES for the regions in Figs. 2(a) and 2(b), respectively. The concentration of Mn, O, and B was lowest at a depth of 50 nm, whereas that of Fe, which indicates the steel sheet substrate, became constant at depths of 50 nm or greater. These results show that the thickness of the oxide layer produced by the ion-plating method was approximately 50 nm for both the Mn–O and Mn–B–O films. Thus, the amount of oxides added to the Mn–O and Mn–B–O films was equal to that present on high-tensile steel.19,20)
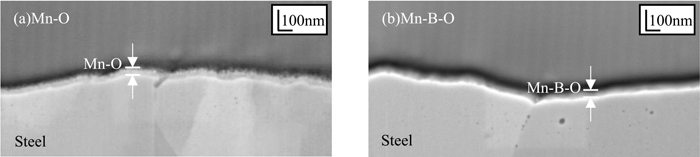
Cross-sectional SEM images of (a) Mn–O ion plating and (b) Mn–B–O ion plating.
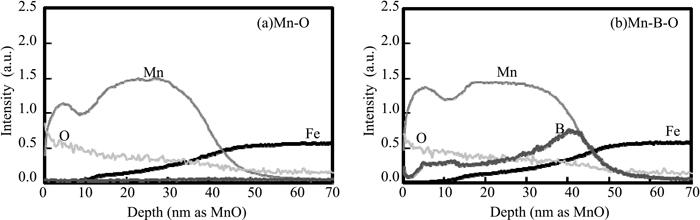
GD-OES depth profiles of (a) Mn–O ion plating and (b) Mn–B–O ion plating.
Figure 4 shows the XRD patterns of the Mn–O and Mn–B–O films formed on the steel sheet surface. Figure 4(a) shows the pattern for the steel sheet substrate before ion plating, and Figs. 4(b) and 4(c) show the patterns for the Mn–O film before and after annealing, respectively. Similarly, Figs. 4(d) and 4(e) show the results for the Mn–B–O film before and after annealing. In the pattern of the steel sheet substrate shown in Fig. 4(a), a strong peak originating from iron was observed at 2θ ≈ 45°. However, the small peak observed at around 2θ ≈ 22° is attributed to the diffraction of half-wavelength X-rays of Cu-Kα by the Fe(110) facet. In the thin-film XRD results before annealing, as shown in Figs. 4(b) and 4(d), peaks were observed at the same positions as those of the steel sheet substrate; however, diffraction peaks originating from the ion plating film were not detected. On the basis of these results, we speculated that the Mn–O and Mn–B–O films were amorphous. However, in the patterns of the annealed samples, as shown in Figs. 4(c) and 4(e), peaks originating from MnO were observed for both the Mn–O film and the Mn–B–O film.
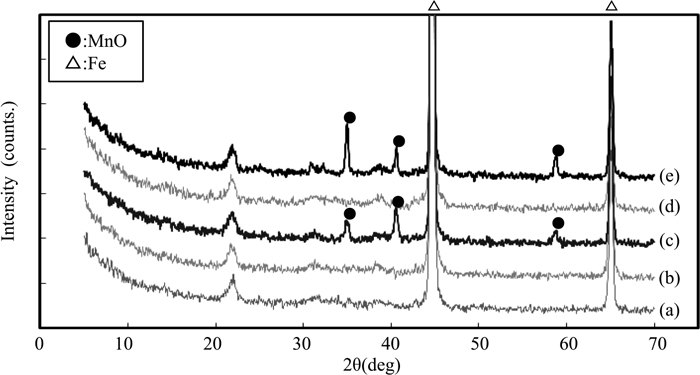
X-ray diffraction profiles (a) before ion plating, (b) Mn–O ion plating before annealing, (c) Mn–O ion plating after annealing, (d) Mn–B–O ion plating before annealing, and (e) Mn–B–O ion plating after annealing.
As described in the previous sections, it was found that both the Mn–O film and the Mn–B–O film, which formed on the steel sheet surface with a thickness of approximately 50 nm, exist in the form of crystalline MnO on the steel sheet surface after annealing. However, the presence of B did not affect the thin-film XRD results. Therefore, to analyze the morphology of MnO in greater detail, surface observation by SEM and an EDS analysis were conducted. The secondary electron images of the Mn–O film after annealing (Fig. 5(a)) show a large number of massive substances with sizes of several tens of nanometers on the steel sheet surface, and the exposed area of the steel sheet substrate was small. In contrast to this, in the case of the Mn–B–O film shown in Fig. 5(b), massive substances of several tens of nanometers and coarse globular substances reaching a size of approximately 500 nm existed on the steel sheet surface, and the exposed substrate region was larger than that on the Mn–O film. Since the EDS analysis results show that Mn and O were detected in the material, as confirmed by surface observation, this suggests that the material was likely MnO, as identified by the thin-film XRD analysis. We also found that, among the MnO on the steel sheet surface with an Mn–O–B film, the intensity of B was high in the coarse globular MnO with a diameter greater than approximately 500 nm.
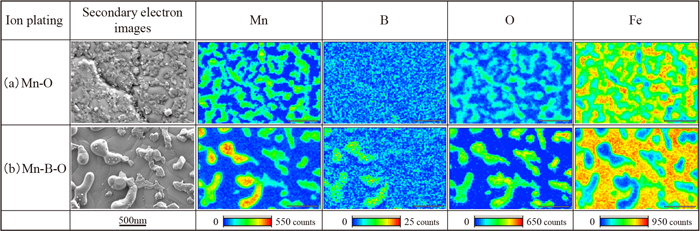
SEM images and EDS elemental mappings of (a) Mn–O ion plating and (b) Mn–B–O ion plating after annealing. (Online version in color.)
Phosphating of the steel sheets with the Mn–O and Mn–B–O films described in the previous section was conducted for 120 s after the annealing, and the coating state of the phosphate crystals on the steel sheet surface was observed. Figures 6(a), 6(b), and 6(c) present the results for the Mn–O film, Mn–B–O film, and original sheet before ion plating, respectively. For the original sheet without ion plating (Fig. 6(c)), phosphate crystals with a diameter of approximately 3 μm covered the steel sheet without gaps on the surface. Next, in comparison with the steel sheet without ion plating, the steel sheet onto which a Mn–O film was added contained 3–5 μm phosphate crystals without gaps on its surface (Fig. 6(a)). On the contrary, rough phosphate crystals with a size of approximately 10 μm were sparsely distributed on the sheet with the Mn–B–O film (Fig. 6(b)), and regions (black arrows) where phosphate crystals had not formed were also observed.
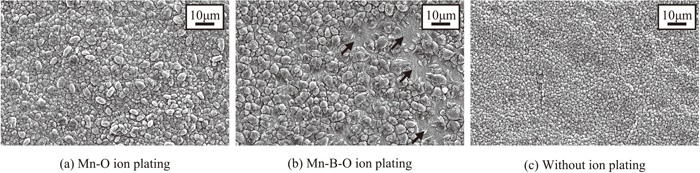
SEM images of (a) Mn–O, (b) Mn–B–O, and (c) without ion plating after phosphating 120 s.
The relationship between phosphating time and the amount of adhering phosphate is presented in Fig. 7. From this figure, the curve representing the adhering amount on the steel sheet with the Mn–O film exhibited approximately the same tendency as that of the reference sheet without ion plating, indicating that phosphate adhesion was saturated at 90 s of phosphating time. On the contrary, in the case of the steel sheet with the Mn–B–O film, phosphate adhesion in the initial stage of phosphating was small in comparison with that of the sheet with the Mn–O film and the sheet without ion plating, and phosphate adhesion was not saturated at 90 s of phosphating time. Therefore, the results show that the phosphatability of the steel sheet with the Mn–B–O film is inferior.
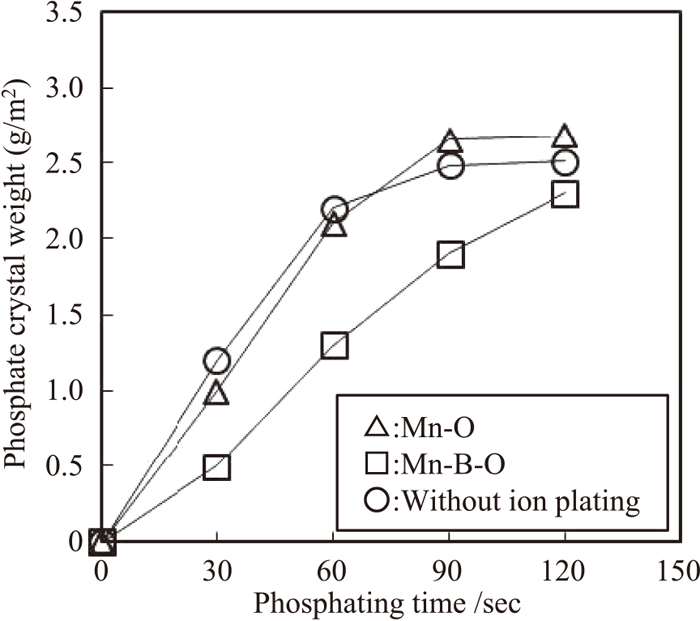
Relationship between phosphating time and phosphate crystal weight.
The aforementioned results clarify the fact that, even with the same amount and same species of manganese oxide, phosphatability differs depending on the morphology of the manganese oxide on the steel sheet surface.
When samples with a Mn–O film without B were annealed on a previously-annealed steel sheet surface (Mn–O film annealed material), fine massive MnO particles with diameters on the order of tens of nanometers were observed. However, coarse globular and fine massive MnO particles with diameters of 500 nm and on the order of tens of nanometers, respectively, were observed on the samples with the Mn–B–O film containing B (Mn–B–O film annealed material) (Fig. 5). The GD-OES analysis results for the Mn–B–O film (Fig. 3(b)) show a peak corresponding to B in the region near the interface between the manganese oxide and the steel sheet substrate, and the SEM-EDS analysis results for the Mn–B–O film annealed material (Fig. 5) show a strong peak due to B in the spectrum corresponding to coarse globular MnO with a diameter greater than 500 nm among the MnO present on the steel sheet surface. These results suggest that coarsening of the MnO occurred during the annealing process and that B possibly contributed to the coarsening of MnO. The equilibrium diagram of a B2O3–MnO two-phase system is shown in Fig. 8.21) The melting point of MnO is 1650°C, which is higher than the annealing temperature (900°C) in the present experiment, whereas the melting point of B2O3 is 577°C, which is considerably lower than the annealing temperature. In addition, it is understood that the melting point of B2O3 was lowered through the formation of a complex oxide with other oxides composed of the other elements.22) On this basis, we presumed that the melting point of the B-containing MnO that formed on the steel sheet surface after annealing was lowered to 900°C or less by the formation of a complex of B2O3 and MnO, which resulted in continuous melting and aggregation of MnO on the steel sheet surface. The results suggest the formation of coarse globular MnO reached approximately 500 nm in diameter. Furthermore, the morphological changes in MnO investigated herein using the ion-plating method induced changes in the addition amounts of Mn and B in the steel. These results are in good agreement with the selective external oxidation behavior observed during annealing.9,10) Hence, we suggest that, if easily oxidized elements, i.e., Mn and B, are added to a steel sheet surface by ion plating and then annealed, the oxidation behavior (melting and aggregation of oxides) observed in the selective external oxidation of the Mn-based high-strength steel sheet surface can be simulated by Mn and B on a mild steel sheet surface.
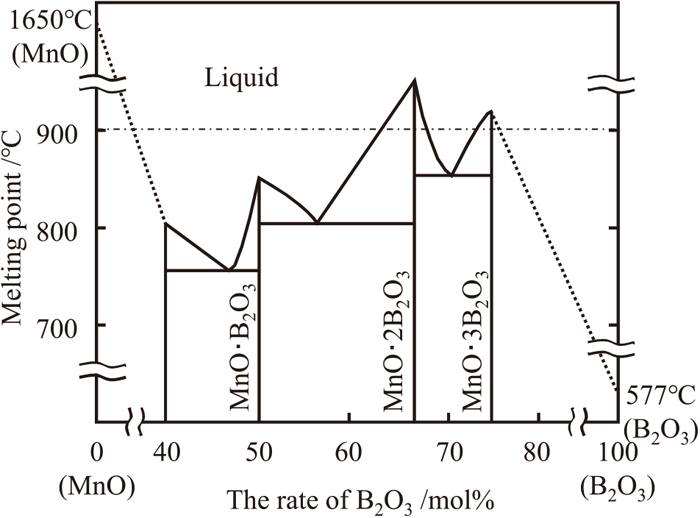
Phase diagram of MnO–B2O3.21)
The results up to this point have suggested the possibility that, when boron is present on the surface of a steel sheet, the melting point of MnO formed on the surface during annealing decreases, resulting in the melting and aggregation of MnO, and finally, the formation of coarse manganese oxide particles (Figs. 4–5). Furthermore, the morphology of the manganese oxide was shown to influence phosphatability under conditions where the amount and kind of the manganese oxide are adjusted (Figs. 5–6). In this section, the relationship between the morphology of the manganese oxide and phosphatability is discussed on the basis of the aforementioned results.
The driving force of the precipitation of phosphate crystals is the dissolution reaction of the steel sheet substrate in the phosphating solution.13) Therefore, it is thought that the main factor that influences phosphatability is likely to be the contact area between the steel sheet substrate and the phosphating solution. From the results in Fig. 5, fine manganese oxide particles were formed on the Mn–O film annealed material surface. Since the steel sheet surface was covered with these manganese oxide particles, the exposed area of the steel sheet substrate was small. Although coarse manganese oxide particles were formed on the surface of the Mn–B–O film annealed material after annealing, the number of oxide particles was relatively small compared with that on the Mn–O film annealed material. Consequently, the exposed area of the steel sheet substrate was large. Given the aforementioned results, good phosphatability was expected with the Mn–B–O film annealed material because of the large contact area between the phosphating solution and the steel sheet substrate. However, the results shown in Figs. 6(a) and 6(b) revealed that the actual phosphatability was poor. Focusing on the initial 30 s in the curves showing the amounts of adhering phosphate crystals (Fig. 7), the adhering amount is as large as 1.0 g/m2 in the case of the Mn–O film annealing material, which had a small exposed substrate area. On the contrary, phosphate crystal adhesion on the Mn–B–O film annealed material was small (0.5 g/m2), or approximately one-half of that on the Mn–O film. On the basis of these results, we presume that the fine manganese oxide particles that formed on the Mn–O film annealed material were dissolved in the initial stage of the phosphating process, and the film then exhibited good phosphatability because of the exposure of the steel sheet substrate. On the contrary, with the Mn–B–O film annealed material, dissolution of the coarse manganese oxide particles in the phosphating solution required a significant amount of time, and as a result, exposure of the steel substrate was difficult and phosphatability was poor. Furthermore, the size of the coarse manganese oxide particles (approximately 500 nm, as shown in Fig. 5(b)) differed from that in the region where phosphate crystals were not formed (approximately several μm, as shown in Fig. 6(b)), and the result was a region where coarse (approximately 500 nm) manganese oxide particles were densely packed on the steel sheet surface. It is thought that this condition resulted in poor phosphatability in this region.
Based on these results, the relationship between the morphology of the manganese oxide and phosphatability is schematically shown in Fig. 9. As shown in Fig. 9(a), in the case of the Mn–O film annealed material, numerous fine manganese oxide particles with diameters of 100 nm or less were present on the steel sheet surface before the phosphating process. In the initial stage of the phosphating process, these fine manganese oxide particles immediately dissolved, exposing the steel sheet substrate. Therefore, after 30 s of phosphating, phosphate crystal nuclei were formed compactly and showed good phosphatability. However, in the case of the Mn–B–O film annealed material, as shown in Fig. 9(b), coarse Mn oxide particles with a size of approximately 500 nm existed partially on the steel sheet surface before phosphating. In the very initial stage (approx. first 10 s) of phosphating, these coarse manganese oxide particles remained without completely dissolving, and after 30 s of phosphating, these particles inhibited the formation of phosphate crystal nuclei and the dissolution reaction of the steel sheet substrate. We propose that this effect was responsible for the poor phosphatability of the Mn–B–O film annealed material.
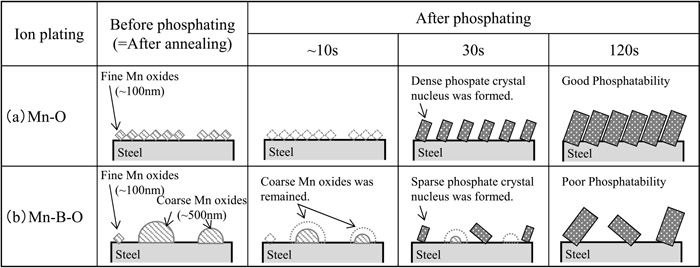
Schematic drawings of steel surface before and after phosphating.
We found that the oxidation behavior (melting and aggregation of oxides) on the surface of manganese-based high-strength cold-rolled steel sheets could be simulated on a mild steel surface by deposition of a manganese oxide film on a mild steel sheet substrate by the ion-plating method and subsequent annealing of the coated sheet. Furthermore, on the basis of our investigation of the relationship between the morphology of the manganese oxide and phosphatability under conditions where the amount and species of the manganese oxide were controlled, the following results were obtained:
(1) When B was present on the steel sheet surface after annealing, coarse globular MnO particles with a diameter of approximately 500 nm were formed. We inferred that these particles were formed by continuous melting and aggregation of MnO because its melting point was lowered by the formation of a complex between B2O3 and MnO during annealing.
(2) Under conditions where the amount and species of the manganese oxide were controlled, it is likely that the morphology of the respective manganese oxides influenced phosphatability. The results indicated that phosphatability was poor when the crystal size of the manganese oxide was large.
The aforementioned results demonstrate the importance of controlling the morphology of monoxides to obtain small particles, in addition to the previously reported method of controlling the species and amount of the respective oxides,19,20) for achieving good phosphatability of Mn-based high-strength cold-rolled steel sheets.