2020 Volume 60 Issue 11 Pages 2512-2518
2020 Volume 60 Issue 11 Pages 2512-2518
The phosphatability of hot-rolled steel sheets has become increasingly important with application of higher strength and thinner steel sheets to automotive parts. Although vanadium (V) is an alloying element which is often added to high strength hot-rolled steel sheets, the effect of V on phosphatability was still unclear. This study investigated the phosphatability of V-added hot-rolled steel sheets by using V-free, 0.20% V, and 0.47% V steel sheets as test specimens. After phosphate treatment, phosphate crystals covered the whole surface of the V-free and 0.20% V steel sheets, but no phosphate crystals were observed on the surface of the 0.47% V steel sheets. In order to clarify this difference, potentiostatic polarization measurement was carried out in the phosphate treatment solution. Phosphate crystals were found on the surface of the V-free steel sheet after both cathodic and anodic polarization. In contrast, no phosphate crystals were found on the surface of the 0.47% V steel sheet after anodic polarization, but similarly to the V-free steel sheet, phosphate crystals had formed after cathodic polarization. A surface analysis by XPS revealed that V oxides had precipitated on the surface of the 0.47% V steel sheet after anodic polarization. V oxidation reaction involves the generation of hydrogen ions and prevent the steel/solution interfacial pH from rising. This reaction identified as the deterioration mechanism of the phosphatability of V-added hot-rolled steel sheets.
The automotive industry is making efforts to reduce the weight of vehicles in order to improve fuel efficiency from the viewpoint of protecting the global environment. At the same time, there is also growing demand for collision safety. In order to achieve both of these goals, the use of high strength steel sheets has been promoted to reduce the thickness of automobile frame parts.1,2,3) In particular, since steel sheets with a thickness of 2.0 mm or more are mainly used in underbody parts, the ratio of underbody parts to the vehicle weight is larger than that of outer body panels. Therefore, application of high strength steel sheets to underbody parts makes an extremely large contribution to vehicle weight reduction. Hot-rolled steel sheets are often used in underbody parts, therefore development of high strength hot-rolled steel sheets is being promoted.
Since many of underbody parts have complicated shapes, excellent formability is required in addition to high strength. In order to achieve both high strength and excellent formability, microalloy elements such as V, Ti, and Nb are often added to high strength steel sheets, as well as Si and Mn are mainly added to them.4,5,6,7,8,9,10,11,12,13) To further increase of the strength, it is necessary to add larger amounts of various alloying elements than in the past.
In addition, corrosion resistance after painting is strongly required in high strength hot-rolled steel sheets, because the allowable thickness reduction may be limited due to the corrosion. It is important to understand the phosphatability of high strength hot-rolled steel sheets because corrosion resistance after painting is greatly affected by the performance of the zinc phosphate treatment14,15) used as a pre-paint treatment.
In high strength cold-rolled steel sheets, it is known that alloying elements affect phosphatability.16) For example, it has been reported that Si and Mn affect the phosphatability of cold-rolled steel sheets.17,18) Since cold-rolled steel sheets are annealed in a reducing atmosphere for Fe, Si and Mn form oxides with a thickness of 1 μm or less on the surface of the steel sheet, and these oxides deteriorate phosphatability. It has also been reported that Cu and Sn deteriorate the phosphatability due to segregation on the steel surface during the annealing process.19)
In contrast, hot-rolled steel sheets are produced in an oxidizing atmosphere for Fe, resulting in the formation of scale with a thickness of several μm or more. Since the scale is removed by pickling, the surface oxide on hot-rolled steel sheets is considered to have less effect on phosphatability than in the case of cold-rolled steel sheets. However, when a larger amount of alloying elements is added to a hot-rolled steel sheet, phosphatability may be affected by the solid solution elements in the steel, but few reports have investigated the effect of solid solution elements on the phosphatability of hot-rolled steel sheets.
Regarding the zinc phosphate treatment reaction, the formation behavior of zinc phosphate crystals when mild steel is polarized anodically and cathodically in a zinc phosphate treatment solution has been reported,20,21) and it explained that complex zinc phosphate treatment reactions can be separated and simplified into anodic and cathodic elementary reactions. Therefore, electrochemical methods are an effective means of understanding the reaction in zinc phosphate treatment.
Focusing on V, this study investigated the effect on the phosphatability of hot-rolled steel sheets, and also investigated the mechanism of the zinc phosphate treatment reaction by using electrochemical methods.
The chemical compositions of the specimens used in this experiment are shown in Table 1. Steels A, B, and C contained the same amount of 0.7 mass% Si and 1.5 mass% Mn, and each had 0 mass%, 0.20 mass%, and 0.47 mass% V, respectively (hereinafter, 0 V steel, 0.20 V steel, and 0.47 V steel). These steels were melted in a lab-scale vacuum melting furnace and cast to 50 kg ingot. The ingots were rolled to 27 mm thick bars and slabs with a thickness of 27 mm were cut off from the rolled bars. After soaking for 3600 s at 1220°C, the slabs were hot-rolled to 2.1 mm thick at a finishing temperature of 900°C. The steel sheets were quenched in water to room temperature after finishing rolling. The obtained hot-rolled steel sheets were mechanically polished to remove the scale on the surface, and finished by mechanical polishing with SiC abrasive paper having a particle size of #800. In order to exclude the effect of differences in surface roughness on phosphatability, in this experiment, the surface was smoothed by mechanical polishing.
| Sample No. | C | Si | Mn | P | S | V |
|---|---|---|---|---|---|---|
| A (0 V steel) | 0.06 | 0.74 | 1.59 | 0.009 | 0.0007 | <0.01 |
| B (0.20 V steel) | 0.06 | 0.70 | 1.47 | 0.010 | 0.0006 | 0.20 |
| C (0.47 V steel) | 0.06 | 0.71 | 1.48 | 0.010 | 0.0006 | 0.47 |
After mechanical polishing, the samples were sheared to 30 mm square, and zinc phosphate treatment was carried out using a surface conditioning agent (Nippon Paint Surf Chemicals Co., Ltd., SURFFINE 5N-10) and zinc phosphate treatment agent (Nippon Paint Surf Chemicals Co., Ltd., SURFDINE EC1000), both of which are conventional products used for automobiles. The zinc phosphate treatment agent is a tricationic treatment agent containing the Zn2+ ion, Mn2+ ion, and Ni2+ ion in addition to phosphoric acid, and contains the NO3− ion as an oxidizing agent.22,23) The pH of the zinc phosphate treatment solution was adjusted to 3.2 by addition of an aqueous NaOH solution. Surface conditioning was carried out by immersing for 30 s, and zinc phosphate treatment was carried out for 120 s, followed by washing with water and drying. The temperature of the surface conditioning solution was 25°C, and the temperature of the zinc phosphate treatment solution was 40°C.
2.3. Polarization Behavior in Zinc Phosphate Treatment SolutionDisk-shaped specimens having a diameter of 16 mm were cut out from the steel sheets after mechanical polishing, and potentiostatic polarization measurement was performed in the zinc phosphate treatment solution at 40°C using an electrochemical measurement system (HOKUTO DENKO CORPORATION, HZ-5000). A silver-silver chloride electrode was used as a reference electrode, and the obtained potential was converted to a value with reference to the standard hydrogen electrode (SHE). The test specimens were immersed in the zinc phosphate treatment solution for 20 s and held at three kinds of potentials, i.e., open circuit potential, potential on the anodic side of open circuit potential (−300 mV vs. SHE), and potential on the cathodic side of open circuit potential (−500 mV vs. SHE) for 600 s, and then washed with water and dried. The open circuit potentials after immersion in the zinc phosphate treatment solution for 20 s were between −379 and −380 mV vs. SHE with little difference depending on the V content in the steel sheets.
Furthermore, the polarization curves were measured in the zinc phosphate treatment solution by the potentiodynamic method. The test specimens were immersed in the zinc phosphate treatment solution for 20 s, and then cathodic polarization was carried out from the open circuit potential to −700 mV vs. SHE at a scan rate of 1 mV/s. Similarly, anodic polarization was performed from the open circuit potential to −200 mV vs. SHE. In these experiments, the test specimens were immersed in the zinc phosphate treatment solution without surface conditioning in order to investigate the reactivity between the steel sheet and the zinc phosphate treatment solution.
2.4. Surface AnalysisThe amounts of V in the steel was analyzed by using the method24) which Kinoshiro et al. has developed, and determined as the solid solution state and as precipitates, respectively. In addition, the element concentration profile in the depth direction from the polished steel surface was measured with a glow discharge optical emission spectrometer (GD-OES, HORIBA, Ltd. GD-Profiler 2). The sputtering depth was calculated from the sputtering rate (60 nm/s for Fe) and the sputtering time.
Surface observation of the steel sheets after zinc phosphate treatment and after potentiostatic polarization using a scanning electron microscope (SEM, JEOL Ltd. JSM-6390A) at an acceleration voltage of 15 keV. The area covered by zinc phosphate crystals was measured from the obtained surface SEM image, and the coverage of the zinc phosphate crystals was calculated by dividing that area by the area of the entire visual field of the SEM image.
The element state on the surface of the specimens was analyzed by using an X-ray photoelectron spectrometer (XPS, ULVAC-PHI, Inc. PHI Quantera SXM) after mechanical polishing, zinc phosphate treatment, and potentiostatic polarization. The X-ray source was Al Kα, and the measurement range was in a diameter of 100 μm. The effect of charging of the specimen was corrected so that the binding energy of the peak of C(1s) was 284.8 eV.
Table 2 shows the V solid solution and precipitation amounts of the 0 V steel, 0.20 V steel, and 0.47 V steel. It was confirmed that almost all of the V added to the steel existed in the solid solution state because water quenching was carried out after finishing rolling.
| Sample No. | Solid-solute | Precipitate |
|---|---|---|
| A (0 V steel) | – | – |
| B (0.20 V steel) | 0.19 | 0.001 |
| C (0.47 V steel) | 0.47 | 0.002 |
Figure 1 shows the element distribution profiles in the depth direction from the steel surface measured by GE-OES. No segregation of V, Si, or Mn was observed on the steel surface in any of the specimens.

GD-OES depth profiles of (a) Steel A (0 V steel), (b) Steel B (0.20 V steel), and (c) Steel C (0.47 V steel). (Online version in color.)
Figure 2 shows the surface SEM images of the specimens with different V contents after zinc phosphate treatment. As shown in Fig. 2(a), the entire surface of the 0 V steel is covered with zinc phosphate crystals having a size of about 2 μm, indicating that the 0 V steel has good phosphatability. In the 0.20 V steel shown in Fig. 2(b), the size of the zinc phosphate crystals is about 3 μm, which is slightly larger than that of the 0 V steel, but the entire surface of the steel sheet is covered, indicating that the phosphatability of the 0.20 V steel was also good. In contrast, in the 0.47 V steel shown in Fig. 2(c), absolutely no zinc phosphate crystals can be observed, and the substrate steel surface is exposed, which shows that the phosphatability of the 0.47 V steel is inferior. From these results, it was found that phosphatability tended to deteriorate with increasing V content in the steel.

SEM images of (a) Steel A (0 V steel), (b) Steel B (0.20 V steel), and (c) Steel C (0.47 V steel) after phosphate treatment.
Figure 3 shows surface SEM images of the steel sheets with different V contents after polarized in a zinc phosphate treatment solution with holding at the open circuit potential, anodic potential, and cathodic potential, respectively. As shown in Figs. 3(a-1), 3(b-1), 3(c-1), granular or rod-like zinc phosphate crystals with sizes of about 10 to 100 μm were observed on all the steels after keeping at the open circuit potential. The amount of precipitated zinc phosphate crystals after keeping at the open circuit potential shown in Figs. 3(a-1), 3(b-1) is smaller than that after the zinc phosphate treatment shown in Figs. 2(a), 2(b). Surface conditioning was not performed in the experiment at the open circuit potential, as a result, the density of crystal formation decreased.14) As shown in Figs. 3(a-2), 3(b-2), 3(c-2), after polarized at the anodic potential, granular zinc phosphate crystals with sizes of about 10 to 50 μm were observed on the 0 V and 0.20 V steels, but no zinc phosphate crystals were observed on the 0.47 V steel. After polarized at the cathodic potential shown in Figs. 3(a-3), 3(b-3), 3(c-3), scale-like zinc phosphate crystals with sizes of about 100 μm had formed on all the steels.
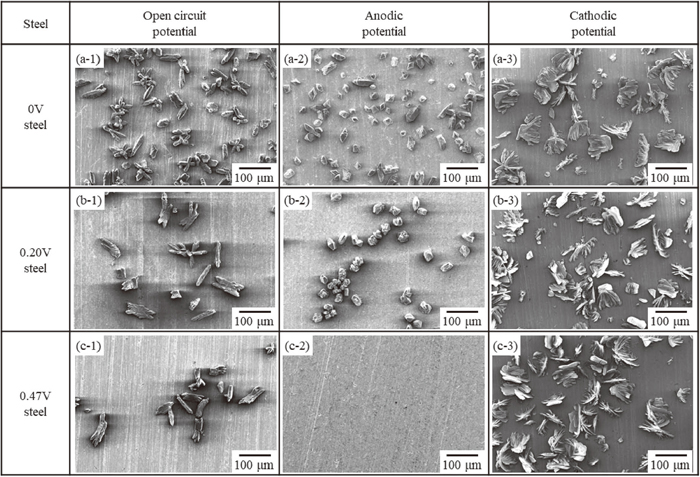
SEM images of (a-1, a-2, a-3) Steel A (0 V steel), (b-1, b-2, b-3) Steel B (0.20 V steel), and (c-1, c-2, c-3) Steel C (0.47 V steel) after polarization in phosphate treatment solution for 600 s at (a-1, b-1, c-1) open circuit potential, (a-2, b-2, c-2) anodic potential (−300 mV vs. SHE), and (a-3, b-3, c-3) cathodic potential (−500 mV vs. SHE).
Next, in order to clarify the contribution of the V content to the anodic reaction and the cathodic reaction in the zinc phosphate treatment reaction, the coverage of the steel surface by the zinc phosphate crystals was obtained from the surface SEM images shown in Fig. 3. The relationship between the V content in the steel and the crystal coverage is shown in Fig. 4. In the case of polarized at the cathodic potential, the crystal coverage was almost the same regardless of the V content. However, in the case of keeping at the open circuit potential and polarized at the anodic potential, the crystal coverage decreased with increasing V content.

Relationship between surface coverage by phosphate crystals after polarization and V content. (Online version in color.)
As shown in Fig. 2, phosphatability tended to deteriorate as the V content in the steel increased. Since V exists in a solid solution state without surface segregation in the steel sheets used in this experiment, the deterioration of phosphatability due to higher V contents might be caused by the solid-solute V in the steel.
In addition, Figs. 3 and 4 indicate that solid-solute V does not affect the zinc phosphate film formation reaction under cathodic polarization in zinc phosphate treatment, but under anodic polarization, the results suggest that solid-solute V does affect the film formation reaction and deteriorates phosphatability.
4.2. Mechanism of Zinc Phosphate Treatment ReactionThe reaction on the steel surface when a steel sheet is immersed in a zinc phosphate treatment solution can be as follows.22)
First, when the steel sheet is contacted with an acidic zinc phosphate treatment solution, dissolution of Fe occurs as an anodic reaction.
| (1) |
| (2) |
| (3) |
| (4) |
| (5) |
As shown in Fig. 3, scale-like zinc phosphate crystals were formed on the steel surface after polarized at the cathodic potential regardless of the V content in the steel. During polarized at the cathodic potential, dissolution of Fe, which is an anodic reaction, does not occur, and since the pH at the steel/solution interface is raised by the cathodic reactions of formulas (2) and (3), the hopeite formation reaction of formula (4) mainly proceeds. The scale-like shape of the zinc phosphate crystals formed after polarized at the cathodic potential shown in Figs. 3(a-3), 3(b-3), 3(c-3) indicates the formation of hopeite. Since this reaction proceeded without dissolution of the steel sheet, zinc phosphate crystals with similar shapes and densities were formed regardless of the V content.
In contrast, in the case of polarized at the anodic potential, a significant difference in the formation of zinc phosphate crystals was observed depending on the V content. In this case, the dissolution reaction of Fe of formula (1) occurs, and since the cathodic reactions of formulas (2) and (3) do not occur, the increase in the pH at the steel/solution interface due to these reactions also does not occur. The zinc phosphate crystal formation reaction shown in formulas (4) and (5) does not proceed unless the interfacial pH increases. However, as shown in Fig. 3(a-2), zinc phosphate crystals are actually formed even after polarized at the anodic potential. Regarding the driving force for the formation of zinc phosphate crystals during anodic polarization, Sato et al.20) reported that during anodic polarization, H2PO4− ions increase and H+ ions decrease at the steel/solution interface due to the influence of the electric field, resulting in an increase in the interfacial pH. Therefore, the crystal deposition reaction proceeds according to Le Chatelier’s principle, as shown in formulas (4) and (5). In this experiment, the zinc phosphate crystals after polarized at the anodic potential shown in Fig. 3(a-2) were granular, indicating that the phosphophyllite formation reaction of formula (5) mainly proceeded. This is because the dissolution of Fe by the anodic reaction proceeded sufficiently and an increase in the pH at the steel/solution interface occurred simultaneously by the aforementioned mechanism.
4.3. Mechanism of Phosphatability Deterioration by Solid-solute VAs shown in Fig. 4, the zinc phosphate crystal coverage decreased after polarized at the anodic potential as the amount of solid-solute V increased. As the possible causes of inhibition of crystal deposition in the anodic reaction by solid-solute V, based on the mechanism of the zinc phosphate treatment reaction described above, it is presumed that the dissolution reaction of Fe is suppressed or the rise in the interfacial pH is suppressed.
In order to clarify the influence of solid-solute V on the dissolution reaction of Fe, polarization curves were measured in the zinc phosphate treatment solution. The results are shown in Fig. 5. The anodic polarization curves indicating the dissolution reaction of Fe showed little difference regardless of the V content. This result means that the dissolution of Fe in the zinc phosphate treatment solution occurs to the same extent regardless of the amount of solid-solute V. The cathodic polarization curves also showed no significant difference depending on the amount of solid-solute V, indicating that the cathodic reaction was not affected by solid-solute V.

Polarization curves of Steel A (0 V steel), Steel B (0.20 V steel), and Steel C (0.47 V steel). (Online version in color.)
Next, suppression of the increase in the interfacial pH is discussed. In order to clarify the state of V on the surface of the 0.47 V steel, the surface state of each test specimen after mechanical polishing, zinc phosphate treatment, and polarized at the anodic potential was analyzed by XPS. The XPS spectrum of V (2p) is shown in Fig. 6. No peak was observed in the steel sheet after mechanical polishing, as shown in Fig. 6(a). However, as shown in Fig. 6(b), after zinc phosphate treatment, peaks indicating the presence of V2O3, V2O4, and V2O5 were observed, and stronger peaks were detected after polarized at the anodic potential, as shown in Fig. 6(c). This result shows that the solid-solute V contained in the steel was dissolved by zinc phosphate treatment and then formed V2O3, V2O4, and V2O5, which precipitated on the surface. Further, since the peaks are strongly detected after polarized at the anodic potential, the deposition reaction of V2O3, V2O4, and V2O5 is considered to be caused by the anodic reaction in zinc phosphate treatment.

V(2p) XPS spectra of Steel C (0.47 V steel) after (a) surface polishing, (b) phosphate treatment, and (c) anodic treatment. (Online version in color.)
Here, the mechanism of the deposition reaction of V2O3, V2O4, and V2O5 is discussed based on the potential-pH diagram. Figure 7 shows the potential-pH diagram of the V–H2O system.25) Considering the reaction near the steel surface, the activity of the ion species in the solution was set to 0.005 in accordance with the V concentration in the 0.47 V steel. When the solution is polarized to the anodic side in the zinc phosphate treatment solution of this experiment at pH 3.2, the solid-solute V undergoes the following reaction and is dissolved in the zinc phosphate treatment solution.
| (6) |
| (7) |
| (8) |
| (9) |
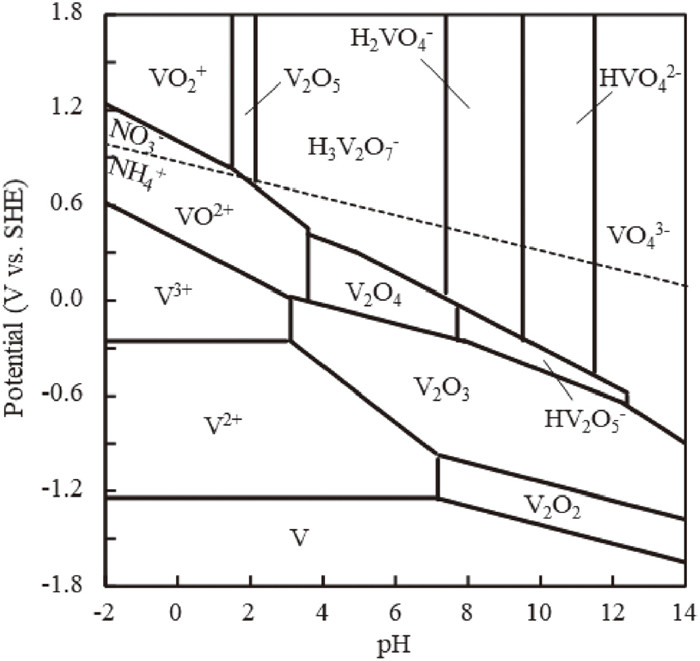
Potential-pH equilibrium diagram for solution system of V–H2O at 298 K (a = 0.005).
Regarding the deterioration of phosphatability by solid solution elements, Usuki et al.26) showed that the phosphatability of Ti-added cold-rolled steel sheets with ground surfaces is poor, and considered the factor causing deterioration to be the inhibition of pH rise due to the formation of Ti oxide accompanied by H+ ion generation during zinc phosphate treatment. It is thought that the phosphatability of the V-containing hot-rolled steel sheets in the present experiment was also deteriorated by a similar mechanism.
Based on the results of this study, the effect of solid-solute V on the zinc phosphate treatment reaction is shown schematically in Fig. 8. In the case of steel sheets containing no solid-solute V, H+ ions are consumed as a cathodic reaction accompanying the anodic dissolution of the steel sheet in the zinc phosphate treatment solution, and the pH at the steel/solution interface rises. Precipitation of zinc phosphate crystals proceeds with this pH rise as the driving force. In the case of steel sheets containing solid-solute V, the solid-solute V is dissolved together with the dissolution of Fe by the anodic dissolution of the steel sheet, forming V2+ ions, which are then oxidized and precipitated as V oxides on the steel surface. The oxide formation reaction is accompanied by the generation of H+ ions, which prevents the increase in pH at the steel/solution interface. This inhibits the formation of zinc phosphate crystals driven by the increase in pH, resulting in deterioration of the phosphatability of the V-containing steel.

Schematic diagrams of mechanism of inhibition of zinc phosphate precipitation reaction by solid-solute V. (Online version in color.)
The effect of V added to hot-rolled steel sheets on phosphatability was investigated using steel sheets with different contents of solid-solute V, and the reaction mechanism was then examined by electrochemical methods. As a result, the following conclusions were obtained.
(1) When the amount of solid-solute V in the steel sheet is 0 mass% and 0.19 mass%, the whole surface of the steel sheet is covered with zinc phosphate crystals after zinc phosphate treatment, but when the amount is 0.47 mass%, phosphatability is deteriorated and no zinc phosphate crystals is observed.
(2) As a result of potentiostatic polarization in the zinc phosphate treatment solution, solid-solute V in the steel sheet inhibited the precipitation of zinc phosphate crystals under anodic polarization.
(3) The solid-solute V in the steel sheet dissolves and is ionized in the zinc phosphate treatment solution, which is followed by an oxide formation reaction accompanied by a pH decrease. This pH decrease inhibits the precipitation reaction of zinc phosphate crystals, which is driven by the increase in pH at the steel/solution interface, and as a result, the phosphatability of the V-containing steel sheet is deteriorated.