2020 Volume 60 Issue 11 Pages 2408-2415
2020 Volume 60 Issue 11 Pages 2408-2415
In this paper, the influence of basicity (CaO/SiO2) on the viscosity and crystallization characteristics of chromium-containing high-titanium slag was studied. The melting temperature and viscosity were measured by employing the hemisphere and rotating cylinder methods, and the composition and morphology of the crystallized phase were analyzed by X-ray diffraction and scanning electron microscopy-energy dispersive spectroscopy. The results showed that the crystallization sequence during the cooling process was spinel, followed by anosovite. The increased basicity led to enhanced softening, hemispherical, and flowing temperatures of chromium-containing high-titanium slag; however, the viscosity significantly decreased. The basicity obviously affected the crystallized phase composition. Furthermore, anosovite was the dominant crystallized phase and a proper level of basicity was beneficial for titanium enrichment.
Vanadium titanomagnetite (VTM) is a multi-element symbiotic mineral that primarily consists of iron, vanadium, and titanium, which provides important comprehensive utilization value.1,2) The available capacity of VTM in China is 9.83 billion tons, and the prospective reserves are more than 30 billion tons.3,4,5,6) Compared with ordinary VTM, Hongge VTM (HVTM) in the Panxi region of China is also rich in chromium but difficult to comprehensively utilize. At present, VTM is mainly smelted by blast furnace (BF) process, but the TiO2 content in the BF slag is low, about 20%–25%. Besides, the titanium in the BF slag exists in the form of perovskite, which is not conducive to titanium extraction.7,8,9,10) In recent years, new processes containing coal have been developed to improve the comprehensive utilization efficiency of VTM, especially the direct reduction process.11,12,13,14,15) However, the recovery rates of valuable elements are still low in these processes. Compared with coal-based direct reduction, gas-based direct reduction has strong competitiveness and potential because of its low environmental pollution, water consumption, and CO2 emission.16,17,18)
To improve the comprehensive utilization efficiency of HVTM, a novel and clean process was proposed by our laboratory.19,20) In this process, HVTM was pelletized and oxidatively roasted, and then reduced in a shaft furnace; subsequently, the reduced pellets were melted and separated for the recovery of valuable elements. Obviously, melting separation is an essential procedure in this process and able to separate metal and slag. The TiO2 content in the separated chromium-containing titanium slag can be as high as 40%, and titanium mainly crystallizes in the form of anosovite. It should be mentioned that viscosity is one of the most important properties of slag, and it is closely related to melting process parameters. Therefore, to realize the efficient separation of metal and slag, it is imperative to study the viscosity of chromium-containing high-titanium slag.
Over the past decades, the viscosity of titanium slag has been widely studied.21,22,23,24,25,26) Shankar et al.21) determined the viscosity of high alumina blast furnace slag by a rotating cylinder method. Kim et al.23) studied the effects of Al2O3 and CaO/SiO2 on the viscosity of blast furnace slag at fully liquid temperatures. However, the titanium content in these studies was relatively low, which was different from chromium-containing high-titanium slag. It is typically understood that the presence of chromium may lead to increased viscosity. In our previous study,27) the influence of TiO2 on the melting property and viscosity of chromium-containing high-titanium slag was investigated. However, compared with other slags, the basicity of chromium-containing high-titanium slag is below 1.0, and it is an important index to ensure smooth operation and efficient separation between metal and slag in the melting separation process. The results presented in previous researchers may not be suitable for the chromium-containing high-titanium slag, which has high levels of titanium and chromium contents. Therefore, to ensure efficient melting separation, it is imperative to have a good understanding of the viscosity and crystallization characteristics of chromium-containing high-titanium slag with different basicities.
In this study, the influence of basicity on the viscosity and crystallization characteristics of chromium-containing high-titanium slag was experimentally determined. First, infrared thermal imaging system was used to record the characteristic temperature. Next, Factsage thermodynamic software was used to predict the crystallized phase composition. Then, the viscosity of the slag was measured by a rotating cylinder method. Finally, the composition and microstructure of the crystallized phase were analyzed. These findings can help to provide theoretical and technical support for the comprehensive utilization of HVTM.
Analytical grade reagents were used as experimental materials, including CaO, SiO2, Al2O3, TiO2, MgO, and Cr2O3. To ensure experimental reliability, the reagents were roasted in a muffle furnace under argon atmosphere at 1000°C for 4 h to remove water and impurities. The roasted reagents were accurately weighed and evenly mixed according to the compositions listed in Table 1.19) To generate more uniform compositions of the slag, 150 g of slag were pre-melted at 1600°C for 1 h. The pre-melted slag was crushed in an agate mortar and then used in subsequent experiments.
| No. | Chemical composition/wt% | CaO/SiO2 | |||||
|---|---|---|---|---|---|---|---|
| CaO | SiO2 | MgO | Al2O3 | TiO2 | Cr2O3 | ||
| 1 | 11.91 | 23.82 | 11.22 | 10.42 | 40.63 | 2 | 0.5 |
| 2 | 13.40 | 22.33 | 11.22 | 10.42 | 40.63 | 2 | 0.6 |
| 3 | 14.71 | 21.02 | 11.22 | 10.42 | 40.63 | 2 | 0.7 |
| 4 | 15.88 | 19.85 | 11.22 | 10.42 | 40.63 | 2 | 0.8 |
| 5 | 16.92 | 18.81 | 11.22 | 10.42 | 40.63 | 2 | 0.9 |
The schematic diagram of the melting point melting rate meter developed by Northeastern University is shown in Fig. 1. The apparatus is composed of a temperature control system, heating system, and track sliding system. The heating element is made of MoSi2, and the furnace temperature is controlled by thyristor with a precision of ±2°C. At first, the pre-melted slag was mixed with a small amount of ethanol to press into a cylindrical shape using a cylindrical mold. Then, the prepared sample was placed on the corundum gasket and moved into the constant temperature zone of the furnace. As the temperature increased at a heating rate of 10°C/min, the sample softened and its height decreased. When the sample heights were 75%, 50%, and 25% of the initial height, the corresponding temperatures were recorded as the softening temperature, hemispherical temperature, and flow temperature, which were defined as Ts, Th, and Tf, respectively.28,29)

Schematic diagram of melting point melting rate. (a) apparatus, (b), (c), (d), and (e) height variation diagram of slag.
The apparatus used for the viscosity measurement is an RTW-10 melt property comprehensive tester that developed by Northeastern University and its schematic diagram is shown in Fig. 2. The apparatus included a rotating system, heating system, circulating cooling system, and measuring system. The rotating spindle was molybdenum and the connecting rod was corundum. U-shaped MoSi2 was used as the heating element, and the temperature was controlled by thermocouple with an error of ±3°C. Before measuring viscosity, the viscometer was calibrated using castor oil at room temperature. The crucible used in the experiment was a graphite crucible. To prevent the reaction between slag and graphite, the graphite crucible was lined with molybdenum sheet.

Schematic diagram of viscometer and dimensions of crucible and molybdenum spindle.
The viscosity measurement was as follows. The crucible with 140 g of pre-melted slag was first placed into the constant temperature zone of the furnace. The furnace was heated at a rate of 10°C/min from room temperature to 1550°C and stabilized at this temperature for 1 h to ensure the slag was uniform and stable. Then, the molybdenum spindle was dipped into the slag to begin the viscosity measurement. During the viscosity measurement process, argon, as a protective gas, was injected at a rate of 3 L/min as controlled by a mass flow controller. When the viscosity reached 5 Pa·s, the spindle was removed from the slag and the viscosity measurement was completed.30,31,32) In addition, the experiments in our study were repeated three times in order to guarantee the accuracy.
2.3. CharacterizationFactSage thermodynamic software was used to predict and calculate the crystallized phase transformation during the slow cooling process. Scanning electron microscopy (SEM, Zeiss, Germany) combined with energy dispersive spectroscopy (EDS) was used to observe the morphology of the crystallized phase. X-ray diffraction (XRD, PANalytical, the Netherlands) equipped with a Cu Kα radiation source was adopted to identify the crystallized phase composition. The diffraction peak was acquired at 40 kV and 40 mA and the scanning angle was 5° ≤ 2θ ≤ 90°. X’Pert High Score Plus software was used for peak analysis.
The composition and content of the crystallized phase were calculated by FactSage thermodynamic software, and the results are shown in Fig. 3. It was found that when the basicity was 0.5, with the decreasing temperature, the crystallized phases were anosovite phase (MgTi2O5), spinel phase (MgCr2O4, MgCrAlO4), sphene phase (CaSiTiO5), pyroxene phase (CaAlSi2O6, CaTiSi2O6, CaMgSi2O6), anorthite phase (CaAl2Si2O8), and rutile phase (TiO2). Titanium primarily existed in the form of anosovite phase and perovskite phase (CaTiO3) was not formed. However, as the basicity increased to 0.6, perovskite phase was formed and crystallized. With the further increase of basicity, the composition of the crystallized phase did not show obvious change until the basicity increased to 0.9. At this level of basicity, the sphene phase disappeared and the olivine phase (Mg2SiO4) crystallized.

Effect of basicity on the crystallized phase composition and content of chromium-containing high-titanium slag. (a) 0.5, (b) 0.6, (c) 0.7, (d) 0.8, and (e) 0.9. (Online version in color.)
It is known that spinel and perovskite are both high melting point phases. When the basicity increased from 0.6 to 0.9, the crystallized content of spinel phase increased from 2.98% to 3.6%. For perovskite, the crystallized temperature increased from 1200°C to 1230°C and the crystallized content increased from 1.45% to 20.1%.
3.2. Melting TemperatureIn this study, the profile change of chromium-containing high-titanium slag during the melting process was recorded by an infrared thermal imaging system. The effect of basicity on the characteristic temperature is shown in Fig. 4. As could be seen from Fig. 4, the basicity had obvious influence on the characteristic temperature of the chromium-containing high-titanium slag. In the experimental range, the three characteristic temperatures of the slag all increased with the increase of basicity. In particular, when the basicity increased from 0.5 to 0.6, the flow temperature increased significantly, from 1272°C to 1326°C, which was an increase of 54°C. When the basicity increased from 0.7 to 0.8, the softening temperature and hemispherical temperature increased obviously, from 1212°C and 1215°C to 1230°C and 1247°C, respectively. From the thermodynamic calculation results shown in Fig. 3, the crystallized content of the high melting point phases, such as spinel and perovskite, increased with the increase of basicity and resulted in the increase of characteristic temperatures of chromium-containing high-titanium slag.

Effect of basicity on the characteristic temperature of chromium-containing high-titanium slag.
In the melting separation process, the melting temperature of slag must be appropriate. If the melting temperature is too high, it will lead to excessive refractory and semi-molten or semi-flowing state. Therefore, in practical industrial production, the melting separation should be optimized to improve the melting temperature and meet the requirements of the furnace condition.
3.3. ViscosityThe effect of basicity on the viscosity of chromium-containing high-titanium slag is shown in Fig. 5. The viscosity decreased as the basicity increased from 0.5 to 0.9 at the same temperature. Notably, the viscosity change was more obvious at lower temperatures. When the temperature was 1550°C, as the basicity increased from 0.5 to 0.7, the viscosity decreased from 0.33 Pa·s to 0.22 Pa·s; however, as the temperature decreased to 1470°C, the basicity had a significant effect on the viscosity, which showed a decrease from 4.87 Pa·s to 3.79 Pa·s. Additionally, it could be found that with the increased temperature, the viscosity decreased due to a weaker interaction among flow units caused by the temperature increase. The viscosity represents the amount of friction force in the molten slag. Temperature and chemical composition are the main factors influencing slag viscosity. The chemical composition primarily affected viscosity by affecting the structure of the slag polymerization.33,34,35)
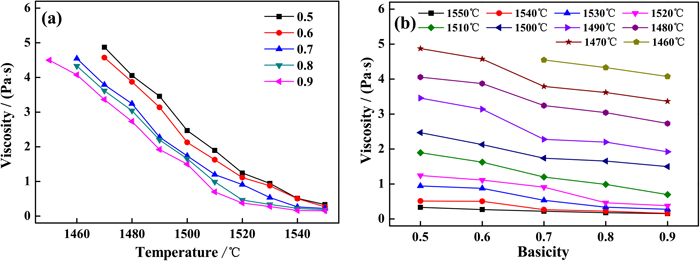
Effect of temperature (a) and basicity (b) on the viscosity of chromium-containing high-titanium slag. (Online version in color.)
Previous studies have showed that SiO2 and Al2O3 form complex network structures, such as [SiO4]-tetrahedron and [AlO4]-tetrahedron through the interconnection of bridge oxygen (O0) in slag,36,37) and the depolymerization of these complex structures into simple structures exhibit a decrease in slag viscosity.38) It is well known that as a basic oxide, CaO can provide free oxygen ions (O2−) in slag, and O2− can react with bridging oxygen (O0) to form non-bridging oxygen (O−), which destroys the complex network structure. With the increase of CaO/SiO2, O2− gradually increases and more O0 is combined, and the structure is depolymerized from a ring to a relatively simple chain structure. Then the chain structure transforms into simpler monomer, dimer, and other small units, as shown in Eqs. (1) and (2):22,39)
| (1) |
| (2) |
Therefore, with the increase of basicity, the slag viscosity decreased and the fluidity was improved.40)
3.4. Determination of the Crystallized PhaseFactSage simulation is a thermodynamic calculation method without kinetic control. The calculated data cannot comprehensively describe the crystallization behavior of slag. To further explore the crystallization composition of chromium-containing high-titanium slag, XRD was conducted and the results are shown in Fig. 6. When the basicity was 0.5, it was observed that the crystallized phases were MgTi2O5, MgAl2O4, CaTiSi2O6, CaMgSi2O6, CaAl2Si2O8, CaTiSiO5, CaTiO3, and TiO2. With the increase of basicity, the main titanium-containing phases changed from MgTi2O5 and TiO2 to MgTi2O5, and the diffraction peak intensities of MgTi2O5 first increased and then decreased. It is worth noting that the diffraction peak intensities of MgAl2O4 and CaTiO3 slightly increased, suggesting that the crystallized content of these phases increased, which was consistent with the thermodynamic calculation. However, some crystallized phase compositions were different with calculated crystallized phase compositions, which might be due to insufficient thermodynamic data of Factsage thermodynamic software in the high titanium system.

XRD patterns of chromium-containing high-titanium slag with different basicities. (a) 0.5, (b) 0.6, (c) 0.7, (d) 0.8, and (e) 0.9.
The crystallized morphology of chromium-containing high-titanium slag is illustrated in Fig. 7. Obviously, there were two zones in each of the SEM micrographs, dark gray was the matrix phase and light gray phase was scattered and distributed in the matrix phase. With the increase of basicity, the morphology of the light gray phase was different. When the basicity was 0.5, the light gray phase was small in size and dense in distribution, and the dark gray phase almost covered the image. When the basicity increased to 0.7, the size of the light gray phase increased and its shape became small chunks and long strips. With the further increase of basicity, the size of the crystallized phase continued to grow. The EDS results indicated that the light gray phase could be identified as a titanium-rich phase. Combined with XRD analysis, it was determined that the titanium-rich phase was anosovite. Point 3 shows that the contents of Ca, Si and O were relatively high, and it could be preliminarily determined that the matrix phase was silicate. The obtained microstructures could help us understand the detailed information of the crystallization process of chromium-containing high-titanium slag and provide reference data for the development of electric furnace melting separation and further extraction processes of valuable elements.

SEM images and EDS spectra of chromium-containing high-titanium slag with different basicities. (a) 0.5, (b) 0.7, (c) 0.9, (d)–(g) points 1–4.
In the melting separation process, the properties of chromium-containing high-titanium slag can affect the melting separation result. The measured properties showed that different basicities could result in property differences of chromium-containing high-titanium slag. As mentioned above, with the increase of basicity, the softening temperature, hemispherical temperature, and flow temperature of chromium-containing high-titanium slag increased, which could be explained by the increased contents of high melting point phases predicted by thermodynamic calculation. The viscosity of chromium-containing high-titanium slag decreased with the increase of basicity, which was more beneficial for the separation of iron from slag. However, if the basicity was too high, it could result in the increase of melting temperature, and perovskite content also increased, which was not suitable for melting separation. Besides, the peak intensities of perovskite in the XRD patterns weakened, and anosovite first strengthened and then weakened, which is not conducive to titanium enrichment in the form of anosovite. From this point of view, these changes were consistent with the thermodynamic calculation.
It is known that the titanium recovery rate is correlated with the acid solubility of the primary titanium-bearing phase in titanium slag. It should be mentioned that anosovite has better acid solubility than perovskite, and a higher proportion of anosovite with less perovskite in the chromium-containing high-titanium slag is beneficial for further titanium extraction process. Therefore, in practical industrial production, considering comprehensively, the basicity of chromium-containing high-titanium slag should be controlled at a suitable range as far as possible to improve the melting separation process and obtain high extraction efficiency of titanium.
The influence of basicity on the viscosity and crystallization characteristics of chromium-containing high-titanium slag was investigated in this paper, and the following conclusions were drawn:
(1) With the increase of basicity from 0.5 to 0.9, the characteristic temperature increased and the trend from softening temperature to flowing temperature became obvious. The viscosity significantly decreased with the increase of basicity. In addition, the viscosity increased with decreasing temperature.
(2) The peak intensity of anosovite phase first decreased and then increased with the increase of basicity. However, the crystallized amount of high melting point phase, such as spinel, increased and resulted in an increased melting temperature.
(3) The basicity had a significant impact on the crystallized morphology of anosovite. With the increase of basicity, the size of anosovite increased and its shape became small chunks and long strips.
This work is financially supported by the National Natural Science Foundation of China (51904066), Fundamental Research Funds for the Central Universities (N182503032), Postdoctoral Foundation of Northeastern University (20190201) and Postdoctoral International Exchange Program (Dispatch Project, 20190075).