2020 Volume 60 Issue 2 Pages 220-225
2020 Volume 60 Issue 2 Pages 220-225
According to the variation of compositions during steel secondary refining process, the ice-quenched samples of CaO–SiO2–FexO–Al2O3 system were prepared, the structure were detected via the method of Raman spectroscopy, and the evolution of structural units were further analyzed. The results showed that, for the Fe3+ cation, two types of units of tetrahedral fourfold coordination ([FeO4]) and octahedral sixfold coordination ([FeO6]) coexisted in the molten slag, and the ratio of [FeO6]/[FeO4] increases with the decreasing ratio of CaO/(SiO2+Al2O3). For the Al3+ cations, four types of aluminum units of Q2Al, Q3Al, Q4Al coexisted in the molten slag and the lower polymerized units Q2Al transform into the higher polymerized Q3Al and Q4Al along with the ratio of Al/(Al+Si) increasing from 0.41 to 0.85. For the Si4+ cations, Q0Si, Q1Si and Q2Si are the main types of [SiO4]-tetrahedral units, the Ca2+ cations of oxygen coordination in [SiO4]-tetrahedral units are gradually replaced by Al3+, which just act as network modifier, with the increase of Al/(Si+Al) ratio. Accordingly, the degree of polymerization of molten slag presented in NBO/T increases with the process of secondary steelmaking.
In modern steelmaking, the LF (ladle furnace) process has been widely used in the secondary refining of liquid steel for its excellent function in adjusting composition and controlling cleanness.1) During the refining process, the refining slag plays an important role in realizing the metallurgical functions such as deoxidation, desulfurization and removing the inclusions of liquid steel. These metallurgical functions are closely related to physical properties such as viscosity and surface tension of refining slag. For example, Sosinsky et al.2) reported that the increasing viscosity resulted in decreasing the fluidity of refining slag, and then hindering to remove the inclusions form liquid steel. Ito et al.3) showed that the lower surface tension was beneficial to foam and thereby affected on the electrothermal efficiency and furnace lifespan of LF. However, physical properties are closely related to the chemical composition of molten slag, which essentially depends on its structure.
Notably, the structural interpretation has been successfully correlated to the physical properties of slag. Kim et al.4,5,6) reported that the viscosity increased with the increasing both the [AlO4]5− tetrahedral units and the three-dimensional AlO2, and decreased with the decreasing of complex Si–O structures and Al–O structures in CaO–SiO2–Al2O3-based slag system. Rajavaram et al.7) also found that the density decreased with increasing Al–O bond length, whereas the density increased due to the formation of the [AlO5] structure. Meanwhile, the structure-surface tension relationship was investigated by Gao8) and Qi,9) where the surface tension decreased with the decreasing of polymerized anions. In addition, Park et al.10) thought that the foaming index also could be correlated to slag structure, and the best-fitting mathematical correlation between the NBO/T value and the foaming index was deduced.
In earlier studies, the structure behaviors of network-forming cations such as Si4+, Al3+ and Fe3+ in molten slag have been studied broadly. Mysen et al.11) revealed that the silicate slags were composed of the [SiO4]-tetrahedron with the different bridging oxygen numbers (QnSi, n being bridging oxygen 1, 2, 3, 4). Subsequently, Sukenaga et al.12) studied structural species such as Q1Si, Q2Si(1Al), Q3Si(1Al) (coupled (Si, Al)-O systems of Al–O tetrahedron and Si–O tetrahedron) were the [SiO4]-tetrahedron existing types, which could be attributed to the role of Al2O3 on CaO–SiO2–Al2O3–(RO or R2O) system. Similarly, Mcmillan et al.13) thought that the aluminate slags were also described by [AlO4]-tetrahedral structure concerning anionic units of Q4Al, Q3Al and Q2Al, and [AlO6]-octahedral structure (α-Al2O3).14) Meanwhile, according to Mysen et al.,15,16) the [AlO4]-tetrahedron display strong preference for three-dimensional network units with increasing Al/(Al+Si) in low-alumina aluminosilicate glasses. At higher amounts of Al2O3 content, the main types of aluminum were Q4Al, Q3Al and Q2Al whereas the remaining aluminum were cations coordinating with oxygen.17) In addition, the distribution of ferric cations among octahedral and tetrahedral sites in alkaline or alkaline earth silicate slags have been studied by Pargamin et al.18) A study of CaO–SiO2–MgO–Al2O3–FexO based on converter slag system was conducted by Wang et al.,19) where the existing Fe3+ in the melt cannot completely form tetrahedral coordination and part of Fe3+ would form octahedral coordination.
In the modern secondary steel refining process, the CaO–SiO2–Al2O3-based slags are widely used, which is commonly transformed from the converter slag system of CaO–SiO2–FexO. During the transformation process, the structural behaviors of Si4+, Al3+ and Fe3+ should change accordingly. In this study, focusing on the change of slag composition during the steel refining process, the variation of structure behavior of cations of Si4+, Fe3+ and Al3+ were investigated, which will contribute to the controlling of the physical properties of the refining slag and thereby improving the quality of the liquid steel.
Figure 1 provides the schematic diagram of compositional changes with respect to the secondary refining, and the compositions of experimental slags as shown in Table 1 were designed according to Fig. 1. The slag samples were prepared using analytical-grade CaO, SiO2, Al2O3 and Fe2C2O4·2H2O, in which the FexO was converted from Fe2C2O4·2H2O. All the oxide chemicals were calcined at 1273 K for 2 h to eliminate moisture, carbonates, and volatile impurities. The raw materials were weighed precisely according to the target compositions listed in Table 1 and then thoroughly mixed in an agate mortar.

Schematic of the compositional changes with respect to the secondary refining.
| Slag | Initial composition (mass%) | Final composition (mass%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CaO | SiO2 | Al2O3 | FexO | CaO/(SiO2+Al2O3) | CaO | SiO2 | Al2O3 | FeO | Fe2O3 | CaO/(SiO2+Al2O3) | |
| No. 1 | 48.75 | 16.25 | 10.00 | 25.00 | 1.86 | 49.30 | 16.29 | 9.84 | 6.39 | 18.95 | 1.89 |
| No. 2 | 49.00 | 11.00 | 25.00 | 15.00 | 1.36 | 50.13 | 11.30 | 23.68 | 4.41 | 10.97 | 1.43 |
| No. 3 | 51.50 | 8.50 | 40.00 | 0 | 1.06 | 51.54 | 8.52 | 39.83 | 0 | 0 | 1.07 |
A 1 g of slag sample mixtures was placed in a beaker-type Pt crucible and suspended in the hot zone of a GCL-01 high-temperature quenching furnace by using molybdenum wire. The schematic diagram is shown in Fig. 2(a). After the temperature was increased to 1873 K (1600°C) and held for approximately 3 hours to reach homogenization. Subsequently, slags were rapidly quenched in ice water to obtain a glass sample. During heating, the heating rising velocity was controlled slower with time goes on, as shown in Fig. 2(b). In addition, the oxygen partial pressure of 10−10 atm was controlled by maintaining a constant flow rate of argon (0.8 L∙min−1, purity >99.9999%), in which condition FexO was considered as the coexistence of bivalent and trivalent irons in the molten slags.20)
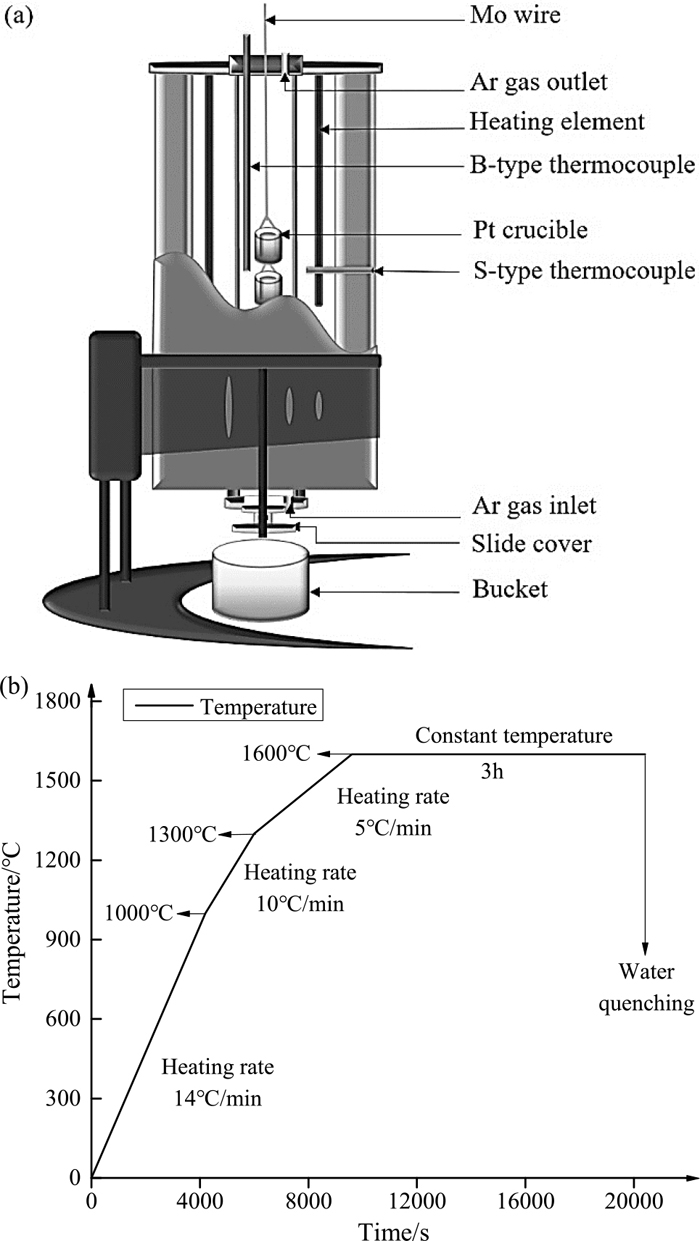
Sketch of high-temperature quenching furnace (a) and temperature-controlling curve (b).
The samples of quenched slag were dried at 120°C for 4 hours, after which ground to below 0.074 mm with an agate mortar. The compositions of quenched slags were confirmed by X-ray fluorescence (XRF) analysis, and the mass ratio of Fe2+/Fe3+ was ascertained by the direct analysis of Fe2+ using the K2CrO7 titration method. XRD was performed on powder with a X’Pert Pro MRD diffractometer and data was collected between 2θ = 0° and 90°. The Raman spectra of quenched glass samples were collected at room temperature in the range 100–4000 cm−1 using an Ar excitation laser source having wavelength 488 nm coupled with Jobin-Yvon Horiba (JY-HR800, France) micro-Raman spectrometer. Raman shifts were measured with a precision of 0.1 cm−1 and the spectra resolution was 0.65 cm−1. After that, the raw data of Raman spectra were imported into Origin 9.0 software, and then smoothed and de-baselined to remove noise and fluorescence effects respectively. The deconvolution of envelope peak was carried out over the range of Raman shifts at 600–1200 cm−1.21,22) The characteristic vibration bands for the Raman spectra of various structural units were determined from established literature shown in Table 2. The spectrum data were fitted by Gaussian function with an aid of the ‘PeakFit’ program within ±0.5% error limit and thus the relative abundance of structural units was calculated by the area fraction of the best-fitted Gaussian curves.11)
| Vibrational Mode | Wavenumber/cm−1 | Ref. |
|---|---|---|
| Asymmetric stretching Si–O− of SiO2 (NBO/Si=0) | 1130–1200 | 20) |
| Symmetric stretching Si–O− of [Si2O5]2− (NBO/Si=1) | 1030–1100 | 15,21,22,23,24) |
| Symmetric stretching Si–O− of [SiO3]2− (NBO/Si=2) | 950–1030 | |
| Symmetric stretching Si–O− of [Si2O7]6− (NBO/Si=3) | 900–920 | |
| Symmetric stretching Si–O− of [SiO4]4− (NBO/Si=4) | 830–890 | |
| Symmetric stretching Al–O−vibration of [AlO4] (Q4Al) | 800–850 | 20,25,26) |
| Symmetric stretching Al–O−vibration of [AlO4] (Q3Al) | 740–790 | |
| Symmetric stretching Al–O−vibration of [AlO4] (Q2Al) | 700–740 | |
| Symmetric stretching Fe–O−vibration of [FeO4] | 650–700 | 19,27,33) |
| Symmetric stretching Fe–O−vibration of [FeO6] | 550–650 | |
| Deformation vibration of Al–O–Al | 560 | 16,17,30) |
| Deformation vibration of Si–O–Al | 430–560 | |
| Deformation vibration of Si–O–Si | 430 |
The results of component analysis are as shown in Table 1. It indicates that the composition of the quenched sample was close to the designed composition. But the content of Fe3+ was slightly higher, which was considered to the oxidation of Fe2+. Since the total iron content and the changing trend of FexO are consistent with the designed value, the influence of deviating composition on structural transformation can be negligible. All the slag samples also confirmed as glassy phase using the X-ray diffractometer (XRD), which indicated glass samples could be representative of high-temperature structure.11) The XRD results are as shown in Fig. 3.

XRD result of as-quenched slag samples at T = 1873 K.
The Raman spectra of CaO–SiO2–FexO–Al2O3 slags are shown in Fig. 4. The spectrum of No. 1 sample is similar to No. 2 sample. They have an obvious strong peak near 670 cm−1, with two weak bands in the 450–550 cm−1 region on the left side of the peak and a weak shoulder in the 800–850 cm−1 region on the right side of the peak. The spectrum of No. 3 sample has two strong peaks near 550 cm−1 and 850 cm−1 respectively, while there are no peaks near 670 cm−1. Comparing No. 1 and No. 2 samples with No. 3 sample, it is indicated that the presence of FexO is the main factor for their differences. Therefore, the peak near 670 cm−1 should be related to the Fe–O stretching vibration.

Raman spectra of CaO–SiO2–FexO–Al2O3 slags.
In addition, comparing No. 1 and No. 2 samples with No. 3 sample, there is also a distinct difference in the high-frequency (700–1200 cm−1) region. The spectra of No. 1 and No. 2 samples have a weak band near 850 cm−1, whereas No. 3 sample has a strong band at 855 cm−1. It can be inferred that the main reason is the addition of Al2O3 in the slag. In aluminosilicate melts, the envelope peak in the high-frequency (700–1200 cm−1) region is the bands related to the vibrations of silicon-oxygen stretching and aluminum-oxygen stretching.15,24,25,26,27) According to previous studies, the bands in the 700–900 cm−1 region are due to aluminum-oxygen stretching vibrations of highly polymerized aluminate structures in alumina-rich aluminosilicate melts.28,29) With the addition of Al2O3 markedly increasing to 40 mass%, the peak intensity over the range of 700–900 cm−1 increases correspondingly.
As seen from the low frequency region, two weak bands are observed at 465 and 497 cm−1. The Raman bands in the 400–600 cm−1 region have been associated with the motions of bridged oxygen in T–O–T linkages, where T is Si4+, Al3+ and Fe3+.16,17,32) The Raman band at 465 cm−1 shifts to lower wavenumber with decreasing CaO/(SiO2+Al2O3) ratio. This Raman band is related to the transverse motion of bridging oxygen within the Si–O–Si linkage.16) A shift to lower wavenumber of the Raman band at 465 cm−1 indicates that the number of Si–O–Si linkage of silicate structure decreases with lower SiO2 content.
However, with the CaO/(SiO2+Al2O3) ratio decreasing from 1.89 to 1.43, the Raman band shifts to higher wavenumber from 497 to 534 cm−1, which is related to the bridging oxygen within the Si–O–Al linkage as discussed by Mcmillan.17) The shift to higher wavenumber of the Raman band at 497 cm−1 indicates that the number of bridging oxygen increases by the decrease of CaO/(SiO2+Al2O3) ratio, viz., the aluminosilicate structure is partly polymerized. Meanwhile, it is found that the relatively of Raman band at 547 cm−1 drastically increases as the CaO/(SiO2+Al2O3) ratio further decreases to 1.07. The Raman band at 547 cm−1 is related to the transverse motion of bridging oxygen within the Al–O–Al linkage.17) Therefore, it indicates that the more complicated aluminum structural units formed.
In order to analyze the structure of molten slags quantitatively, the Gaussian deconvolution of Raman bands in the CaO–SiO2–FexO–Al2O3 slags has been carried out and the results are shown in Fig. 5. The Raman bands in the 600–700 cm−1 region was related to the Fe–O stretching vibration in the above discussion. As seen from Fig. 5, the bands are observed at approximately 607 and 671 cm−1 of No. 1 sample and at approximately 637 and 675 cm−1 of No. 2 sample, respectively. According to previous studies, Fe3+ is an amphoteric cation and can occupy both tetrahedral and octahedral sites.31,34) The peaks near 671 and 675 cm−1 were identified as the symmetric stretching vibration of tetrahedral coordination of Fe3+. The peaks at approximately 607 and 637 cm−1 were identified as the symmetric stretching vibration of octahedral coordination of Fe3+.30,33) With the CaO/(SiO2+Al2O3) ratio in slag decreasing from 1.89 to 1.43, the peak intensity of [FeO6] increases, and the value of [FeO6]/[FeO4] increases. The increasing [FeO6]/[FeO4] ratio indicates that Fe3+ cation displays strong preference for forming octahedral coordination (only acts as network modifier). Presumably, Fe3+ and Al3+ in the melts may behave as an amphoteric ions, the state of Fe3+ ion seems to be changed with the percentage of Al2O3 in the slag increases, that is, the Al3+ cations can substitute for the fourfold coordinated polyanions of Fe3+ to form Al3+ polyanions, resulting in an increase of DOP. When Al3+ ion is located in the tetrahedral site, it needs one O2− ion and one electric charge. To satisfy this configuration, O2− has to be supplied from a basic oxide such as CaO.35) However, Ca2+ ions are not fully supplied for slags with a high Al2O3 content due to the scarcity of Ca2+. In this case, more [FeO6] would be formed to substitute Ca2+ to act as the network modifier.19)

Deconvolution results of Raman spectra and relative area fraction of each structural units.
When Al/(Al+Si) is 0.41, the Raman band in the medium frequency region is observed at approximately 731 cm−1, which is assigned as the Al–O stretching vibration in [AlO4]-tetrahedral units with the NBO/Al=2 (Q2Al). Along the value of Al/(Al+Si) increases from 0.41 to 0.85, the bands with the Al–O stretching vibration of NBO/Al=1 (Q3Al) and NBO/Al=0 (Q4Al) are observed one after another.13) With increasing Al/(Al+Si) ratio, the fraction of polymerized aluminate unit Q2Al decreases, and the fraction of the polymerized aluminate units Q3Al and Q4Al increases. It indicates that the polymerized Q3Al units and fully polymerized Q4Al units are formed by the simple Q2Al units.
Apart from the role of Al3+ as a network former aforementioned, Al3+ can probably acts as a cation coordinated with the oxygen of [SiO4]-tetrahedron. Based on Mysen et al.’s studies,11) in aluminosilicate glass, the Si–O stretching motions modified by A13+ ions appeared in the high frequency region. As can be seen from the convolution results, the three bands at 825 cm−1, 894 cm−1 and 966 cm−1 in the lower Al2O3 content of No. 1 sample are relative to the symmetric stretching vibrations of silicate tetrahedra with four, three and two oxygens bound to calcium. It is considered that these silicate units respectively correspond to Si(OCa)4, -Si(OCa)3 and =Si(OCa)2. Their Raman bands shift to 868 cm−1, 920 cm−1 and 982 cm−1 with increasing Al/(Al+Si) ratio, and then shift to 895 cm−1, 965 cm−1 and 1021 cm−1. According to Mcmillan et al.,17) three peaks at 895 cm−1, 965 cm−1 and 1021 cm−1 in aluminosilicate units correspond to Si(OAl)4, -Si(OAl)3 and =Si(OAl)2, respectively. Therefore, it is suggested that the three peaks at 868 cm−1, 920 cm−1 and 982 cm−1 are the bands of Si(OCa)2(OAl)2, -Si(OCa)2(OAl) and =Si(OCa)(OAl), respectively. The possible types of the symmetric stretching vibration in silicate tetrahedra are shown in Fig. 6. In addition, the fraction of Q2Si unit decreases and the fraction of Q0Si unit increases, due to the increasing basicity providing O2− and depolymerizing the silicate network structure. However, because the silica tetrahedron slightly changes, it has little effect on the degree of depolymerization of molten slag.

Schematic of the proposed high-frequency vibrations responsible for aluminosilicate band systems.
NBO/T is commonly used to measure the degree of polymerization of silicate melt. The theoretical NBO/T can be calculated by formula (1) based on the chemical compositions of slag samples. If the theoretical NBO/T exceeds 4 (the maximum of NBO/T is considered to be 4 in the melt structure), it will be specified as 4. The experimental values of NBO/T can be calculated by formula (2) based on the deconvolution results of Raman spectra. Because neither Fe3+ nor Al3+ has Raman scattering coefficients corresponding to its tetrahedral structures and the results calculated by using the molar fraction and the area fraction of silicon structural units have little deviation, the area fraction is regarded as the molar fraction for calculation. In addition, when the theoretical NBO/T are calculated, three conditions were considered: both Si4+ and Al3+ cations form tetrahedrons, both Si4+ and Fe3+ cations form tetrahedrons, and all the cations of Si4+, Al3+ and Fe3+ form tetrahedrons. The calculation results of non-bridge oxygen number n(NBO/TSi+Al), n(NBO/TSi+Fe), n(NBO/TSi+Fe+Al) and n(NBO/Texp) are shown in Fig. 7.
| (1) |
| (2) |

Comparison of nominal and experimental values of n(NBO/T).
Comparing the theoretical and experimental values of n(NBO/T) in Fig. 7, it is found that, on the conditions of Al/(Al+Si) ratio are 0.41 and 0.71, n(NBO/TSi+Fe+Al)<n(NBO/Texp)<n(NBO/TSi+Al), viz. the measured non-bridged oxygen number is between the theoretical non-bridge oxygen number assuming both the cations of Si4+ and Al3+ form tetrahedron and all the cations of Si4+, Al3+ and Fe3+ form tetrahedron, which indicates that some Fe3+ cations act as the network modifier except forming tetrahedral coordination. Similarly, on the conditions of Al/(Al+Si) ratio are 0.71 and 0.85, n(NBO/TSi+Al+Fe)<n(NBO/Texp)<n(NBO/TSi+Fe) and n(NBO/TSi+Al)<n(NBO/Texp), some Al3+ cations just act as network modifier except forming [AlO4]-tetrahedron.
Figure 7 also shows that, the ratio of NBO/Texp decreases with the changing of refining slags, that is to say, the degree of polymerization of slag increases. The reason is that the fraction of aluminate units Q3Al and Q4Al with more complex structure and higher degree of polymerization in molten slag significantly increase during the secondary refining process of steel.
Existing forms of slag structure are 4- and 6-coordinated iron-oxygen structure, aluminum-oxygen tetrahedron and silicon-oxygen tetrahedron in the molten slag. These structures are all transformed during secondary refining of steels, the NBO/T value of the melt decreases and degree of polymerization increases.
The value of [FeO6]/[FeO4] increases with the decrease of CaO/(SiO2+Al2O3) ratio, and Fe3+ cation displays strong preference for the octahedral coordination in the melt. The simple Q2Al units are formed to the polymerized Q3Al units and fully polymerized Q4Al units with increasing Al/(Al+Si) ratio. The [SiO4]-tetrahedron structural composition of the structure species Q0Si, Q1Si and Q2Si changes, which the Al3+ in the non-bridged oxygen coordination cations increases with the increase of Al/(Al+Si) ratio.
This work was supported by the National key R & D Program of China (Grant No. 2017YFC0805100), the National Natural Science Foundation of China (Grant No. 51674069, 51974075) and the Fundamental Research Funds for the Central Universities of China (Grant No. 182506001).