2020 Volume 60 Issue 7 Pages 1401-1408
2020 Volume 60 Issue 7 Pages 1401-1408
To enhance the descent of droplets in the coke bed of a blast furnace, the sliding angle of the water droplet and the advancing and receding contact angles at the time of sliding were measured. Coke used as a reducing agent in a blast furnace was employed as a substrate. Because the shape of the coke surface varies with the gasification reaction with CO2, the coke substrate was treated with heat (1273 K) in a CO/CO2 atmosphere. Irregularities of approximately several micrometers were formed on the coke surface by the gasification reaction, and the sliding angle of the droplet decreased.
Coke is charged with iron ore from the top of the blast furnace to maintain the permeability of the moving bed in the furnace. A coke bed is formed between the cohesive zone and the deadman at the bottom of the blast furnace, and the molten iron and slag formed at the cohesive zone descend to the coke bed. However, the hot blast gas blown from the tuyeres rises through the vacancy in the coke bed, so a counterflow of liquid and gas occurs. When hold-up of liquid is formed in the packed bed, liquid fills the space of the bed, and, thus, gas permeability decreases. Enhancement of the descent of the liquid droplet in the coke bed is therefore desirable. Because the hold-up of liquid remarkably decreases the gas permeability of the packed bed, many studies have been carried out to investigate this phenomenon. Formulas for the amount of hold-up in a packed bed have been reported,1,2) obtained in experiments using a cold model. Ohgusu et al. derived a formula of static hold-up of CaO–SiO2–Al2O3–MgO melt from experiments at a higher temperature.3) It was shown by a numerical simulation that the amount of static hold-up depends on the wettability.4) When the liquid wets the solid of the packed bed, a liquid hold-up is formed in the vicinity of the contact point of packed bed materials.5,6) However, when liquid does not wet the solid, liquid hold-up forms in the bottleneck of the vacancy in the packed bed.7,8) As described above, the hold-up of the liquid is influenced by the surface tension, the wetting angle, and the packing-layer structure.
For a system similar to the wetting phenomena of coke and slag, measurements on the surface tension of molten oxides and the wettability of molten oxides with carbonaceous materials have been reported.9,10) In recent years, measurement of the contact angle of slag and coke considering the influence of the ash on the wetting phenomena has been done.11,12) The contact angle of coke and molten iron was reported to be 60°–130°,13) and it is influenced by concentrations of C and S in the iron. In addition, the contact angle of the melt of the CaO–SiO2–Al2O3 system and carbonaceous material has a wide range of 20° to 160°, and that is varied with contacting time and reaction. In systems containing Fe2O3, the contact angle is decreased by the reduction reaction. However, slag containing no iron oxide is not wet with carbonaceous material, and the angle is more than 90°.14) In addition to the effect of ash, the wetting of coke and molten slag and iron are influenced by the surface properties, reaction at the interface, and motion state of the liquid.
As described above, the relationship between the contact angle and the hold-up in the packed bed and the static wettability on the smooth carbonaceous material has been extensively studied; however, for considering the droplet motion in the coke-packed bed, it is necessary to investigate the behavior of droplets in packed a bed with a surface that is not smooth.
The relation between the wettability and the falling angle of droplets has been studied in the field of surface science.15,16,17,18,19,20,21,22) Many studies have been conducted on the lotus and rose petal effects caused by the hierarchical roughness. However, the falling and flow of droplets in a packed bed of coke composed of a nonuniform and nonsmooth surface have not been sufficiently studied.
In a previous work, the relationship between the transfer resistance of triple circles around droplets on coke and the surface structure of coke with the gasification reaction was reported.23) The relationship between the sliding angle and the dynamic contact angle of the droplets staying in the packed bed was not sufficiently examined in that study.
In the present study, considering the motion of molten slag and iron on the coke surface, the relationship between the sliding angle and dynamic contact angles of water droplets on a nonsmooth coke surface was investigated. The surface structure of coke was modified by a gasification reaction with CO2. The relationship between the surface structure and sliding angle was investigated.
Metallurgical coke used in blast furnace operation is employed as a material of substrate. The composition of the coke substrate obtained through a proximate analysis is shown in Table 1. Coke was cut as substrates with dimensions of 40 × 30 × 4 mm. The surface was polished with #400 emery paper. A mullite tube of φ40 (O.D.) × 600 mm was installed as a reaction tube to a horizontal electric resistance furnace. The atmosphere in that was controlled with a gas mixture of N2/CO/CO2 using mass flow controllers.23) Coke was placed in the hot zone in the furnace, heated to 1273 K in N2, and then reacted with CO2 gas in a CO/CO2 = 1/1 mixture at 1273 K. The gas flow was 200 ml/min, and the holding time was 2, 4, or 8 h. The substrate was then removed from the furnace and cooled in a N2 stream. In the heat treatment, the gasification reaction of carbon progresses at the surface of the coke, and, thus, the surface structure changes.
| mass% | |||
|---|---|---|---|
| Fixed carbon | Volatile | Ash | Water |
| 87.66 | 0.26 | 12.08 | 0.28 |
According to the above procedure, samples of various conditions of gasification reaction as unreacted coke and reacted coke for 2, 4, and 8 h in a CO/CO2 atmosphere were prepared, and the surface of the sample was observed. The relationship between gasification reaction condition and reaction ratio of coke was reported elsewhere.23) Water wets with the coke and penetrates the inside of the coke. In order to form droplets and clarify the influence of surface shape on dynamic contact angle, fluorine-based water repellent (FLUORO SURF) was impregnated, while care was taken not to change the surface shape of the sample; it was dried for 24 h at room temperature. As a flat and smooth sample, a glass plate with a water-repellent material was employed. Pure water was used as the liquid sample.
2.2. Analysis of Surface Structure of CokeBefore subjecting the sample to water-repellent treatment, the surface structure of the sample was observed using electron probe micro analyzer (EPMA) and an optical microscope capable of measuring the surface shape of the sample. The fine surface condition and element distribution were measured by EPMA, and the optical microscope was used for observation of the surface roughness and sharpness.
2.3. Measurement of Sliding Angle and Advancing and Receding AnglesAs shown in Fig. 1, a metal stage was installed on a smoothly rotatable table with a horizontal axis, and a clip for fixing the coke substrate was placed on it. To capture the shape of the specimen on the tilted coke, the camera was installed at the height of the stage in the direction of the rotation axis. An arbitrary amount (10 to 100 ml) of distilled water was placed on the coke from the top side to form droplets. While photographs were taken with the camera, the tilt of the stage was increased slowly until the droplet fell. As shown in Fig. 2, the slope α of the substrate at the time of falling, the advancing contact angle θA, and the receding contact angle θR of the droplet just before the fall were measured, respectively, based on the image taken. The static contact angle and contact surface radius of the droplet on the horizontal substrate were also derived.

Schematic of experimental apparatus. (Online version in color.)

Measurement of dynamic angles. (Online version in color.)
Figure 3 shows the relationship between weight and sliding angle of a water droplet on a water-repellent glass plate. The sliding angles were, respectively, 70° and 30° for droplets of approximately 0.01 and 0.05 g, and the sliding angle decreased with increasing droplet weight. The advancing and receding contact angles at this fall are shown in Fig. 4. The static contact angle of water on this substrate was approximately 100°. However, the advancing contact angle was slightly larger, and the receding contact angle was smaller. It was also found that contact angle hysteresis, which is the difference between the advancing and the receding contact angles, was large in the range of 0.02–0.04 g, where the weight of the droplet was small, and the hysteresis decreased with increasing weight of the droplet. This might be a result of the balance of the contact width and drop weight, the relation between droplet size and hysteresis is discussed in 3.2.2.

Sliding angle of water droplet on flat substrate.

Advancing and receding contact angles of water droplet on flat substrate.
The relationship between the weight of the water droplet and the sliding angle is shown in Fig. 5. Figures 5(a)–5(d) indicate the result for coke with a gasified reaction for 0, 2, 4, and 8 h, respectively. The sliding angle decreased with increasing droplet weight on all substrates. Comparing nongasified and gasified coke, the sliding angle of the water droplet on gasified coke is significantly small.

Relationship between weight of droplet and sliding angle. (a) Non-heat treated, (b) 2 h, (c) 4 h, (d) 8 h.
Figure 6 shows advancing and receding contact angles just before sliding. Figures 6(a)–6(d) indicate the result on coke with a gasified reaction for 0, 2, 4, and 8 h, respectively. The change in advancing and receding angles due to the size of the droplet is small. The advanced contact angle on unreacted coke is clearly larger than the receding contact angle; however, the difference of angles is small on coke with the heat treatment. This difference corresponds to the force which the droplet receives at the time of falling.

Relationship between weight of droplet, and advancing and receding contact angles. (a) Non-heat treated coke, (b) 2 h, (c) 4 h, (d) 8 h.
The relationship between the weight of the droplet and the sliding angle on smooth glass, unreacted coke, and coke with gasification reactions for 2 and 8 h is shown in Fig. 7. Figures 6 and 7 show that the hysteresis of the contact angle on the gasified coke was small, and the sliding angle on the coke was smaller than on glass and nontreated coke. Moreover, the sliding angle on unreacted coke was larger than that on a smooth substrate.

Comparison of sliding angles on various substrates.
The sliding angle of the droplet on the smooth surface can be calculated from the advancing and receding contact angle of the droplet by using a formula proposed by Furmidge. Moreover, by rearranging it for the sliding angle, it can be written as in Eq. (1).
| (1) |
Figure 8 shows the relationship between the contact angle hysteresis and the sliding angle of the water droplet on the water-repellent glass, untreated coke, 2-h-gasified coke, and 8-h-gasified coke. The straight line in the figure represents a line taking the value of γgl/g of 0.0074 [g/mm] following Eq. (1) as a slope. The value was obtained using the surface tension of water and gravitational acceleration.

Relationship between hysteresis of contact angle and sliding angle.
The measured value and the value obtained from Eq. (1) are close to each other. However, focusing on the result for the non-heat-treated coke, when the hysteresis is large, the sliding angle by measurement tends to be slightly larger than the line following Eq. (1). The effect of surface structure on the relationship is not clear. Overall, because there is no considerable difference in the relationship between the hysteresis and sliding angle owing to the difference in substrate, it is shown that the relationship of Eq. (1) can be applied to some extent regardless of the surface texture.
3.3. Evaluation of Variation of Coke Surface by Gasification ProcessThe appearances of samples with different gasification treatments are shown in Fig. 9. Figures 9(a), 9(b), and 9(c) are substrates with no treatment and heat treatment for 2 and 8 h, respectively. As the gasification time becomes longer, the surface of the coke sample turns a brighter color.

Appearance of coke substrates after heat treatment. (a) No-heated (b) heated for 2 h (c) heated for 8 h. (Online version in color.)
Figure 10 shows the coke sample surface observed by scanning electron microscopy. Figures 10(a) and 10(b), respectively, show the surface of untreated coke and the coke with gasification treatment for 2 h. Pores of various sizes coexist in Figs. 10(a) and 10(b) from several hundred micrometers to pores of 10 μm or less. Regarding the distribution of pores of about several hundreds of micrometers, there is no clear difference between Figs. 10(a) and 10(b); however, in Fig. 10(a), smooth parts can be visually observed among vacancies and holes, while, in Fig. 10(b), fine irregularity exists in the portion corresponding to the smooth parts in Fig. 10(a). This fine irregularity is attributable to the fact that the surface properties have formed as a result of the progress of the following reaction.
| (2) |

SEM images of coke surface. a) non-heat treated, b) heated in CO/CO2 for 2 hs.
Figures 11(a) and 11(b) show the concentration of elements of the surface of the untreated coke and that gasified for 2 h observed by EPMA, respectively. Panels show a secondary electron image, backscattered electron image, and concentration of C, Si, S, and O near the sample surface. Comparing Figs. 11(a) and 11(b) shows that concentrations of Si and O were increased, and the gangue particles are exposed on the surface by the gasification process for coke. This phenomenon is one factor that causes changes in the surface properties of the coke.
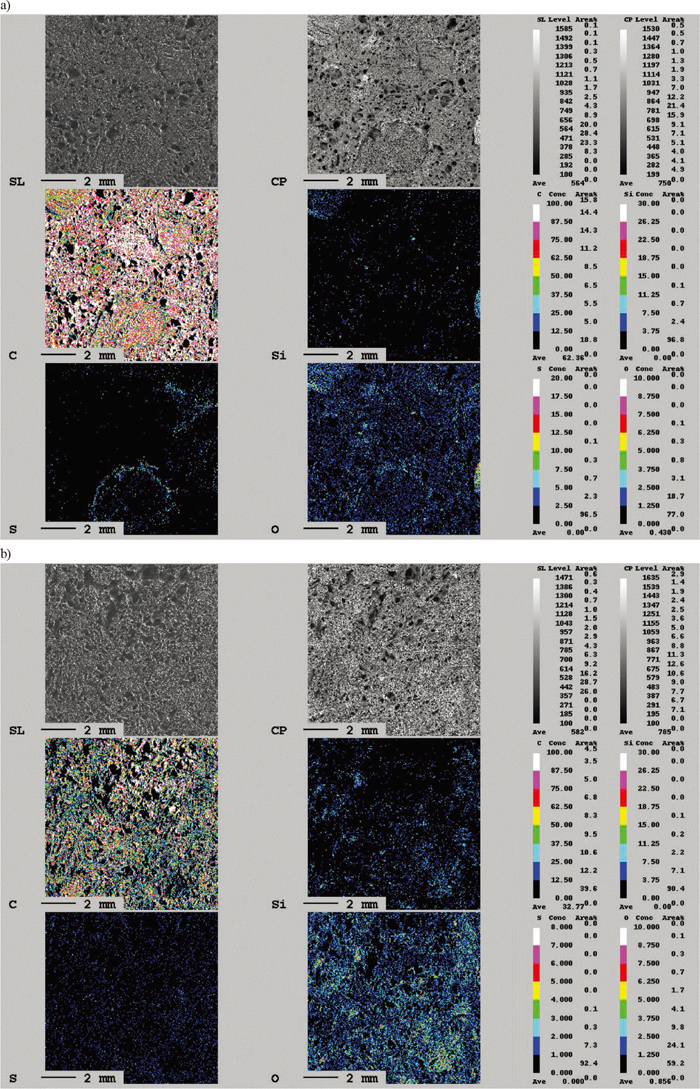
EPMA observation of surface of coke. a) non-heat treated, b) heated in CO/CO2 for 2 hs. (Online version in color.)
A stereoscopic image of a certain area on the surface of the coke substrate was taken with a digital microscope, and the surface shape was measured. Figure 12 shows an example of a stereoscopic image of the surface shape of the coke without heat treatment. For the three arbitrary straight lines on the substrate surface shown in the figure, the surface roughness on each line was analyzed. Three arbitrary lines avoiding large pores were selected, and the results of line analysis were averaged to obtain the roughness of the surface texture. In this analysis, two kinds of objective lenses of 10 times and 20 times different magnification ratios were used, and the observation regions were 1960 × 1960 and 245 × 245 μm. Here, for a region of 1960 × 1960 μm, a stereoscopic image was formed with a pitch of 12.1 μm in the vertical direction, and the pitch was 0.859 μm in the vertical direction for the region of 245 × 245 μm.

Position of measurement of surface roughness on coke substrate. (Online version in color.)
Figures 13(a), 13(b), and 13(c), respectively, show the analysis results of a line of 1960-μm width in the nonreacted coke substrate and those with gasification reactions for 2 and 8 h. There was a difference in shape depending on the measurement position. With large irregularities, there was more than a 100-μm variation in the depth direction, while fine changes of a few micrometers were not detected. The surface roughness was evaluated by the arithmetic average of the roughness profile, Ra (μm). Ra was derived according to JIS B 0601. The reference length was 1960 μm of the observation width. In this experiment, the Ra of the measurement position of each substrate in the 1960-μm-width region was 18.8 μm for unreacted coke, 22.1 μm for 2-h-gasified coke, and 17.8 μm for 8-h-gasified coke. Ra was strongly influenced by the presence of large irregularities and pores, and the resolution in the measurement in this region corresponds to 12 μm. Therefore, in this analyzing region, the variation in Ra because of a fine irregularity by gasification reaction cannot be evaluated.

Surface profiles of coke for 1960 μm width. a) non-heat treated, b) heated in CO/CO2 for 2 hs and c) heated in CO/CO2 for 8 hs. (Online version in color.)
Figures 14(a), 14(b), and 14(c), respectively, show the results of analysis of a line of 245-μm width in the nonreacted coke substrate and those with gasification reactions for 2 and 8 h. There was a difference in shape depending on the measurement position. The surface of coke without a gasified reaction seems smooth; meanwhile, numerous irregularities with a depth of approximately 5 μm exist on the substrate subjected to a gasified reaction. Moreover, irregularities of approximately 5 μm were formed on the surface of the inner side of larger pores. The Ra of each substrate was 0.7 μm for untreated coke, 2.0 μm for 2-h-gasified coke and 6.6 μm for 8-h-gasified coke. The roughness of the relatively smooth part of the surface increased with the gasification reaction time depending on the resolution of the analysis. The coke surface was formed from pores and irregularities of different sizes, so the evaluation of the roughness varied considerably depending on the resolution and the sampling position at the time the surface roughness was measured. The change in the surface roughness that influences the wettability due to the gasification reaction on the coke surface was difficult to evaluate with irregularities of more than 20 μm; however, that changes in the range of Ra = 0.7–6.6 μm.

Surface profiles of coke for 245 μm width. a) non-heat treated, b) heated in CO/CO2 for 2 hs and c) heated in CO/CO2 for 8 hs. (Online version in color.)
As shown in Fig. 7, a large sliding angle was observed on the coke without heat treatment as compared with on the smooth glass surface. However, the sliding angle on the coke with heat treatment became small. On the surface of the non-heat-treated coke, larger pores of more than 100 μm were also observed, and the average roughness was 18 μm. However, because the average roughness of a relatively smooth position including no large pore was 0.7 μm, the value of the roughness varied significantly depending on the position to be evaluated. As with the results in the previous sections, no change in the structure of larger pores was observed by the heat treatment, but the fine roughness of the relatively smooth part increased to 6.6 μm. The fine irregularity of the surface significantly decreased the sliding angle.
On the surface of the non-heat-treated coke, the scale of the unevenness of the smooth portion was small, and it was clearly different from the part with larger pores. Extrand reported that the roughness has no effect on the sliding angle on a smooth substrate with Ra of approximately 0.2 μm at the maximum, but that the nature of the surface and the contact angle determine the sliding angle;20) therefore, it can be assumed that the roughness of the smooth part may have a small influence on the sliding angle, and, with regard to the smooth portion on the untreated coke, a falling behavior similar to that for the smooth glass surface would be exhibited. However, the presence or absence of large pores was different between the non-heat-treated coke and the glass surface, and the large pores on the coke surface would increase the sliding angle in this experimental result. As described above, the fine irregularity and the large pores on the coke surface have different influences on the sliding angle of the droplet. For a detailed understanding of the liquid flow in the coke-packed bed, further research is required on the size of the pores that has an effect on the falling behavior.
In this study, the relationship between the sliding angle of droplets and the dynamic contact angles of water droplets on coke substrate was investigated. The influence of the shape on the coke surface on the sliding angle and the dynamic contact angle was obtained.
(1) The relationship between the sliding angle and hysteresis of contact angles on a smooth surface was established for that on the surface of coke.
(2) The contact angle hysteresis of a droplet on coke without a gasification reaction was larger than that on a smooth surface, and that on coke with a gasification reaction for more than 1 h was smaller than on a smooth surface.
(3) Fine irregularities on the scale of several micrometers were formed on the coke surface by the gasification reaction of coke.
(4) After fine irregularities were formed, the hysteresis of the contact angle decreased as the gasification reaction time of coke increased; however, the change was small.
Authors thanks to the member of research group Mechanisms and Control of Cohesive Zone Phenomena for Blast Furnace Permeability of ISIJ, for their appropriate advices.