2020 Volume 60 Issue 9 Pages 1878-1885
2020 Volume 60 Issue 9 Pages 1878-1885
In this study, the effects of the addition of Ce and Ti on the evolution of inclusions in Al deoxidized Fe-17Cr-9Ni austenitic stainless steel were investigated. When the Ce was added before Ti, Al2O3 can be effectively modified into Ce-containing oxides by Ce. With the increase of Ce content, the oxides evolutionary process is as follows: Al2O3 → CeAl11O18 → CeAlO3 → Ce2O3. After the addition of Ti, TiN appeared on the surface of oxides except Ce2O3. When Ti was added before Ce, due to the simultaneous effects of Ti on the modification of smaller size Al2O3 and precipitation of TiN, spherical Al2O3–TiOx inclusion that was completely wrapped by TiN formed. On the other hand, the larger size Al2O3 was not significantly modified by Ti, and cannot completely be wrapped by TiN. After Ce was added, the oxides which were not completely wrapped by TiN were modified to Ce-containing oxides. However, Ce could not modify the oxides that were completely wrapped by TiN.
Ti-stabilized austenitic stainless steel has been widely used in various industrial applications, such as chemical plants, power plants and pressure vessels in gas-cooled nuclear reactors.1) The good mechanical properties and corrosion resistance make it perform well in the wicked environment,2,3,4) and according to previous research, Al2O3, TiOx, Al2O3–TiOx and TiN were the main inclusions after the addition of aluminum and titanium to austenitic stainless steel.5,6) Such inclusions were seriously affect the continuous casting process and final steel product quality.7,8) Therefore, in order to reduce the impact of inclusions, it is necessary to control the concentration and size of inclusions in the molten steel.
The effect of rare earth elements on properties of steel products has been studied by many researchers.9,10,11) Rare earth elements have a strong affinity for oxygen and sulfur in the molten steel, so the addition of rare earth element can effectively modify oxides and sulfides.12,13,14) Moreover, the rare earth inclusions can effectively improve the quality of steel product since their thermal expansion coefficients are similar with steel matrix. Ce as a common rare earth element was often added to steel as an additive to improve material properties. The resistance of oxidation and creep of 253 MA steel at high temperature can significantly improved after the addition of Ce.15) Ce also could improves the weldability of weld metal by refining the grains.10,16) After the addition of Ce, the pitting resistance and intergranular corrosion resistance of stainless steel under the aggressive environment could be effectively improved.17,18,19) Moderate addition of rare earth elements also plays an important role in improving the performance of steel product.20) When the content of Ce was between 0.01% and 0.1%, the Ce-bearing austenitic stainless steel exhibits good antibacterial properties.21) However, excessive addition of Ce can also has a harmful effect on steel products by affecting the type and quantity of inclusions.22,23)
Many researchers had investigated the evolution process of inclusions after the adding Ce to molten steel.24,25,26,27) Katsumata et al. found that four oxides of Al2O3, Ce2O3, CeAl11O18 and CeAlO3 were produced after adding Ce in Fe-25mass%Cr-6mass%Ni steel, and the amount of Ce-rich oxides increased with the increase of Ce content.24) Wang et al. studied the modification process of Al2O3·MgO spinel by the addition of Ce in the spring steel. They found that the spinel present in the steel was wrapped by Ce-rich inclusion after the addition of Ce.25) Huang et al. investigated the effect of rare earth on the formation of carbonitrides in H13 steel. They found that the Ce oxides and oxysulfides inhibited the precipitation of carbonitrides during solidification of molten steel.26) However, previous researchers have only studied the effect of Ce on oxides and nitrides separately. Few researchers have studied the effect of adding Ce to the molten steel in which contained both oxides and nitrides, especially when the oxide was wrapped with TiN. The modification mechanism of Ce to heterogeneous inclusion composed of oxides and nitrides was not clear.
In the present work, Fe-17Cr-9Ni austenitic stainless steel was used as a raw material to investigate the effect of Ce content on inclusions. Combined with thermodynamic calculations and laboratory experiments, the evolution mechanism of inclusions after Ce and Ti addition in Al deoxidized Fe-17Cr-9Ni austenitic stainless steel was elucidated.
The experiments were carried out in a Si–Mo heating tube furnace. In each experiment, approximately 180 g steel was contained in an MgO crucible (30 mm ID, 80 mm high) surrounded by an alumina protection crucible. After the crucible was placed in a vertical tube furnace, the high pure argon was used as a protective gas to protect the sample from oxidation by air, and the flow rate of argon was 300 ml/min. After the argon was introduced for 30 min, the resistance furnace began to heat and the temperature was measured with a B-type thermocouple. After sample was completely melted, the temperature was maintained at 1873 K for half an hour to achieved homogenization of the chemical composition, and then Al, Ce and Ti were added to molten steel according to a predetermined scheme. Figure 1(a) shows the detailed procedures of heats A, B and C. During these three heats, Ce was added two minutes after deoxidation of Al. The first sample was taken 10 min after Ce addition by a quartz tube and quenched in the water. Just after taking the first sample, titanium was added. Subsequently, the steels were respectively held for 2 min and 12 min at 1873 K and then sampled by a quartz tube, followed by rapid quenching (No. 2 sample and No. 3 sample). Figure 1(b) shows the detailed procedures of heats D and E. In these two heats, Ti was added to the molten steel 2 min after adding Al, and finally Ce was added. Sample 1 was taken 10 min after the addition of Ti, and samples 2 and 3 were obtained 2 min and 12 min after the addition of Ce, respectively. Each sample was rapidly quenched by water after taken out from crucible.
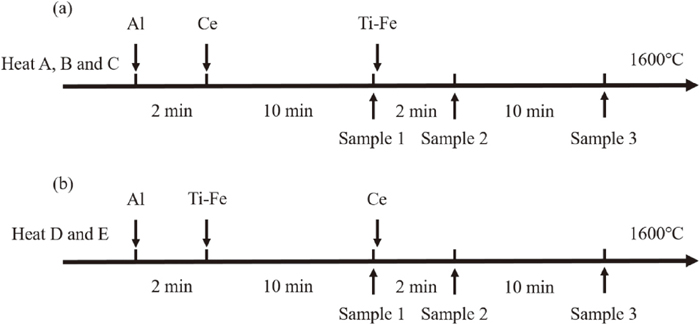
Schematic illustration of melting and sampling procedure: (a) heat A, B and C; (b) heat D and E.
The experimental steel was Fe-17Cr-9Ni austenitic stainless steel, which was taken from the AOD stage of producing AISI 321 stainless steel in a compact process (electric arc furnace (EAF) → argon oxygen decarburization (AOD) → ladle furnace (LF) → continuous casting (CC)). Table 1 shows the compositions of the experimental steel. The other raw materials adopted in the present work were Al wire (99.9 mass% Al), Ce metal (99.9 mass% Ce), Ti–Fe alloy (22.55 mass% Fe, 70 mass% Ti, 0.3 mass% C, 0.7 mass% Si, 1 mass% Mn, 0.01 mass% P, 0.04 mass% S, 0.3 mass% Cu, 5 mass% Al).
| C | Si | Mn | P | S | Cr | Ni | T.O | T.N |
|---|---|---|---|---|---|---|---|---|
| 0.023 | 0.37 | 1.06 | 0.035 | 0.001 | 17.39 | 9.06 | 0.0081 | 0.0123 |
The contents of Al, Ce and Ti in the melts of five heats were detected by inductively coupled plasma emission spectrometry method. The total oxygen and total nitrogen contents of samples were analyzed using inert gas fusion-infrared absorptiometry method. The compositions of sample 3 in each heat were showed in Table 2. The quenched samples used for inclusions analysis were prepared by cutting, grinding and polishing. Scanning electron microscope (SEM) and energy dispersive spectroscope (EDS) were used to analyze the morphology and the compositions of inclusions in each sample. In this paper, inclusions described as “X + Y” denote that there are two separate X and Y phases in one heterogeneous phase inclusion, while “X - Y” denotes a homogeneous phase inclusion composed of two X and Y components.
| Heat | Al | Ce | Ti | T.O | T.N |
|---|---|---|---|---|---|
| A | 0.056 | <0.0005 | 0.24 | 0.0073 | 0.0166 |
| B | 0.056 | 0.0012 | 0.27 | 0.0081 | 0.0176 |
| C | 0.057 | 0.005 | 0.22 | 0.0093 | 0.0160 |
| D | 0.036 | 0.012 | 0.21 | 0.0069 | 0.0129 |
| E | 0.07 | 0.025 | 0.26 | 0.0082 | 0.0124 |
In heat A, B and C, after Al deoxidation, Ce was added to the molten steel to modified Al2O3 inclusions, and finally Ti–Fe alloy was added. The difference between heat A, B and C was the addition of different amounts of Ce. In heat A, the content of Ce in the molten steel was less than 0.0005 mass%, while in heat B and C, the content of Ce in the molten steel reached 0.0012 mass% and 0.005 mass%. According to the results of SEM and EDS, there were many inclusions of the same type in the samples of heat A and B, so the inclusions in heat A and B were discussed together.
The typical inclusions found in the samples of heat A and B are shown in Fig. 2. Figure 2(a1) shows the homogeneous Al2O3 inclusion found in sample A1. Figure 2(a2) shows the heterogeneous Al2O3 + CeAl11O18 inclusion and such type of inclusions was found in both samples A1 and B1. The element mapping of heterogeneous Al2O3 + CeAl11O18 is showed in Fig. 3(b1). It can be seen that CeAl11O18 was on the outer layer of the Al2O3 + CeAl11O18 inclusion. Figures 2(a3) and 2(a4) show the homogeneous CeAl11O18 and heterogeneous CeAl11O18 + CeAlO3 inclusion, respectively. These two types of inclusions were only found in sample B1. The element mapping of heterogeneous CeAl11O18 + CeAlO3 is showed in Fig. 3(b2). It can be seen that the oxide (CeAlO3) with high Ce content was completely wrapped by the oxide (CeAl11O18) with low Ce content. After the addition of Ti–Fe alloy, Ti-containing inclusions appeared in the molten steel. Figure 2(a5) shows Al2O3–TiOx inclusion wrapped by TiN, such type of inclusions was only found in samples A2 and A3. The inclusions types of Al2O3 + CeAl11O18 wrapped by TiN are shown in Fig. 2(a6). These types of inclusions were found in all samples after taken adding Ti–Fe alloy to heat A and B. Figures 2(a7) and 2(a8) show the CeAl11O18 and CeAl11O18 + CeAlO3 oxide wrapped by TiN, respectively. These types of inclusions were found in samples B2 and B3. Figures 3(b3) to 3(b6) show the element mapping of typical inclusions after Ti addition in heat A and B.
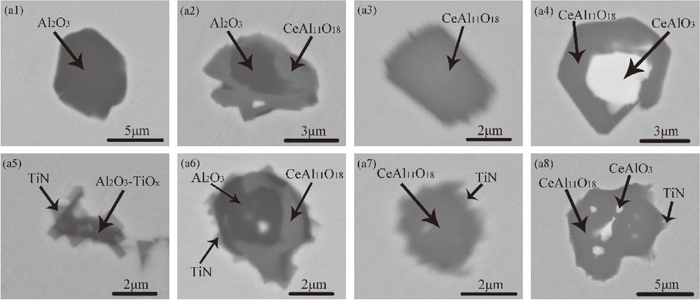
Morphology and composition of typical inclusions in heat A and B. (a1) Al2O3 in sample A1; (a2) Al2O3 + CeAl11O18 in sample A1; (a3) CeAl11O18 in sample B1; (a4) CeAl11O18 + CeAlO3 in sample B1; (a5) Al2O3–TiOx + TiN in sample A2; (a6) Al2O3 +CeAl11O18 + TiN in sample A2; (a7) CeAl11O18 + TiN in sample B2; (a8) CeAl11O18 + CeAlO3 + TiN in sample B2.
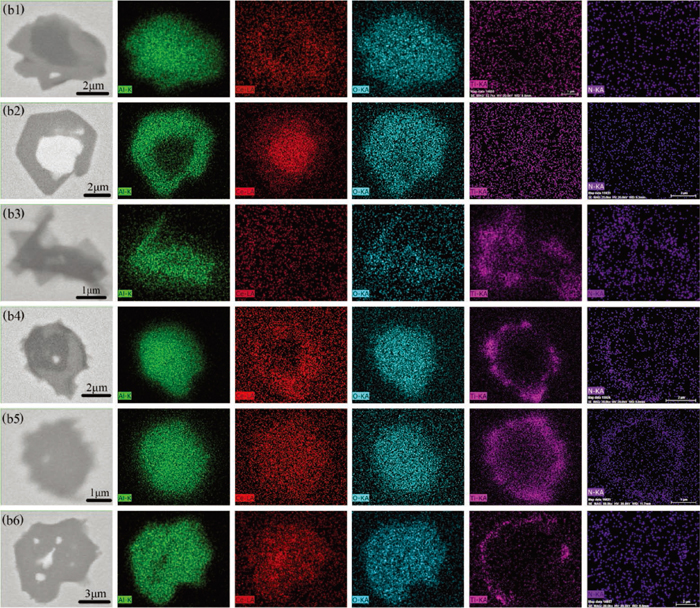
Elemental mapping of a typical heterogeneous inclusions in heat A and B. (b1) Al2O3 + CeAl11O18 in sample A1; (b2) CeAl11O18 + CeAlO3 in sample B1; (b3) Al2O3–TiOx + TiN in sample A2; (b4) Al2O3 + CeAl11O18 + TiN in sample A2; (b5) CeAl11O18 + TiN in sample B2; (b6) CeAl11O18 + CeAlO3 + TiN in sample B2. (Online version in color.)
Figure 4 shows the typical inclusions found in the samples of heat C. Figures 4(c1) to 4(c3) show typical inclusions in sample C1, these three types of inclusions were homogeneous CeAl11O18, heterogeneous CeAl11O18 + CeAlO3 and homogeneous CeAlO3 inclusions. The element mapping of heterogeneous CeAl11O18 + CeAlO3 inclusions is shown in Fig. 5(d1). After the addition of Ti–Fe alloy, TiN appeared on the surface of the original oxide. Figures 4(c4) to 4(c6) show the oxides of CeAl11O18, CeAl11O18 + CeAlO3 and CeAlO3 which were wrapped by TiN. These titanium-containing inclusions were typical inclusions in samples C2 and C3. The element mapping of these three typical inclusions is shown in Figs. 5(d2) to 5(d4).
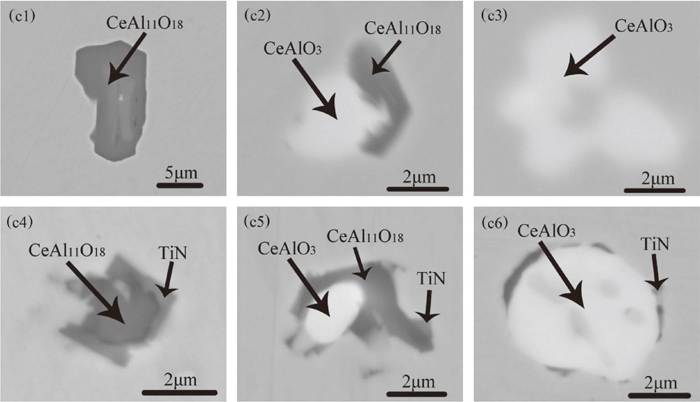
Morphology and composition of typical inclusions in heat C. (c1) CeAl11O18 in sample C1;(c2) CeAl11O18 + CeAlO3 in sample C1; (c3) CeAlO3 in sample C1; (c4) CeAl11O18 + TiN in sample C2; (c5) CeAl11O18 + CeAlO3 + TiN in sample C2; (c6) CeAlO3 + TiN in sample C2.
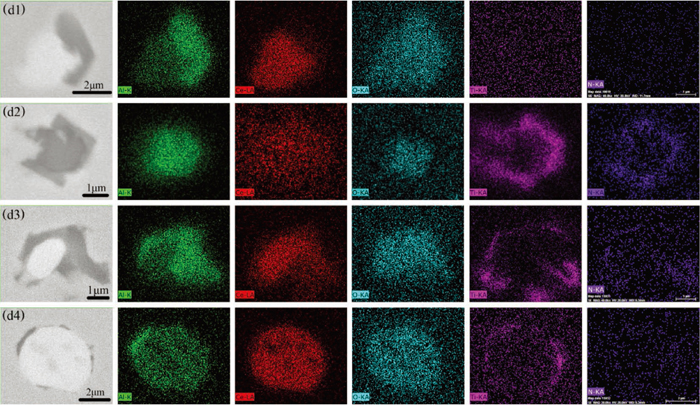
Elemental mapping of a typical heterogeneous inclusions in heat C. (d1) CeAl11O18 + CeAlO3 in sample C1; (d2) CeAl11O18 + TiN in sample C2; (d3) CeAl11O18 + CeAlO3 + TiN in sample C2; (d4) CeAlO3 + TiN in sample C2. (Online version in color.)
In heat D and E, after Al deoxidation, Ti–Fe alloy was added to the molten steel before Ce. Figure 6 shows the typical inclusions found in the samples of heat D and E. After the addition of Ti–Fe alloy, two typical types of heterogeneous inclusions were found in samples D1 and E1: Al2O3 + TiN inclusion (Al2O3 was not completely wrapped by TiN, as shown in the Fig. 6(e1)) and Al2O3–TiOx + TiN inclusion (Al2O3–TiOx was completely wrapped by TiN, as shown in the Fig. 6(e2)). It can be seen that the size of Al2O3 + TiN inclusions was larger than the Al2O3–TiOx + TiN inclusions. The element mapping of these two typical heterogeneous inclusions is shown in Figs. 7(f1) and 7(f2). After the addition of Ce, a large amount of Ce-containing inclusions appeared in the molten steel. Ce2O3 was a type of typical inclusion found in both samples E2 and E3, as shown in the Fig. 6(e3). Figure 6(e4) shows the homogeneous Ce2O3–CeS inclusion, which was only found in sample E2. Figure 6(e5) shows a heterogeneous multi-layered CeAl11O18 + CeAlO3 + TiN inclusion, the core of the inclusion was CeAl11O18, the middle layer was CeAlO3, and the outer layer was TiN. Figure 6(e6) shows the CeAlO3 + TiN inclusion that the CeAlO3 core was wrapped by TiN. Both CeAl11O18 + CeAlO3 + TiN and CeAlO3 + TiN were the two typical inclusions found in samples D2, D3, E2 and E3. The inclusion shown in Fig. 6(e7) was heterogeneous inclusions found in sample E3. The inner layer of the inclusion was Ce-containing oxysulfide, the middle layer was CeAlO3, and the outer layer was TiN. Figures 7(f3) to 7(f5) show the element mapping of the heterogeneous inclusions composed of TiN and Ce-containing oxides.
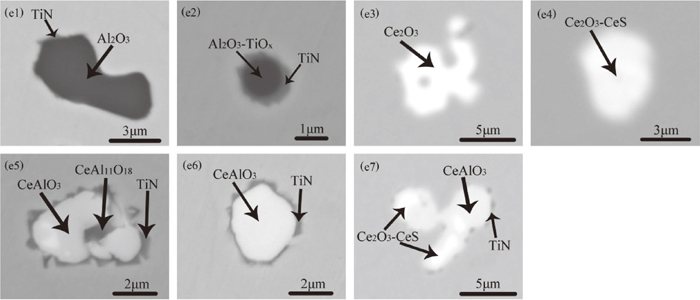
Morphology and composition of typical inclusions in heat D and E. (e1) Al2O3 + TiN in sample D1; (e2) Al2O3–TiOx + TiN in sample D1; (e3) Ce2O3 in sample E2; (e4) Ce2O3–CeS in sample E2; (e5) CeAl11O18 + CeAlO3 + TiN in sample D1; (e6) CeAlO3 + TiN in sample D1; (e7) Ce2O3–CeS + CeAlO3 + TiN in sample E3.
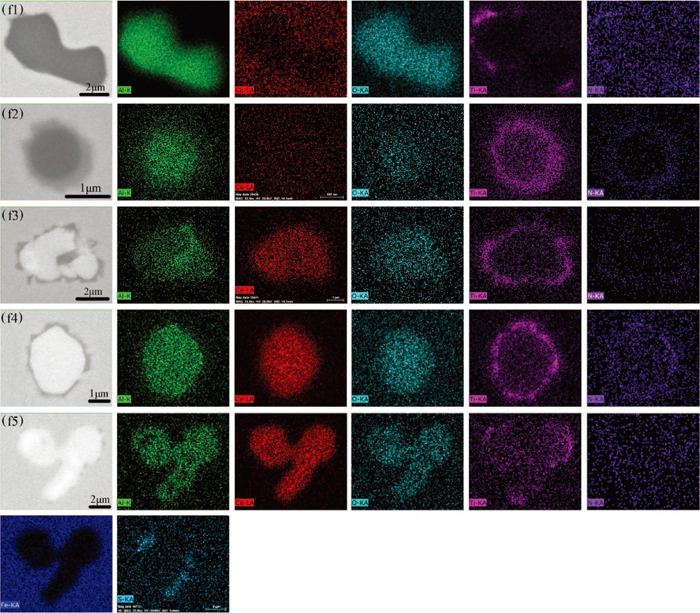
Elemental mapping of a typical heterogeneous inclusions in heat D and E. (f1) Al2O3 + TiN in sample D1; (f2) Al2O3–TiOx + TiN in sample D1; (f3) CeAl11O18 + CeAlO3 + TiN in sample D1; (f4) CeAlO3 + TiN in sample D1; (f5) Ce2O3–CeS + CeAlO3 + TiN in sample E3. (Online version in color.)
After Al deoxidation, a large amount of Al2O3 was generated in the molten steel. In order to study the effect of Ce addition on Al2O3 inclusions, Factsage 7.3 software was used to calculate the evolution of inclusions with the increase of Ce content in the Fe-17Cr-9Ni-Mn-0.5Si-0.001S-0.05Al-0.008O steel. The results are shown in Fig. 8. It can be seen that as the Ce content in the molten steel increases, the inclusions gradually change to Ce-rich oxides.
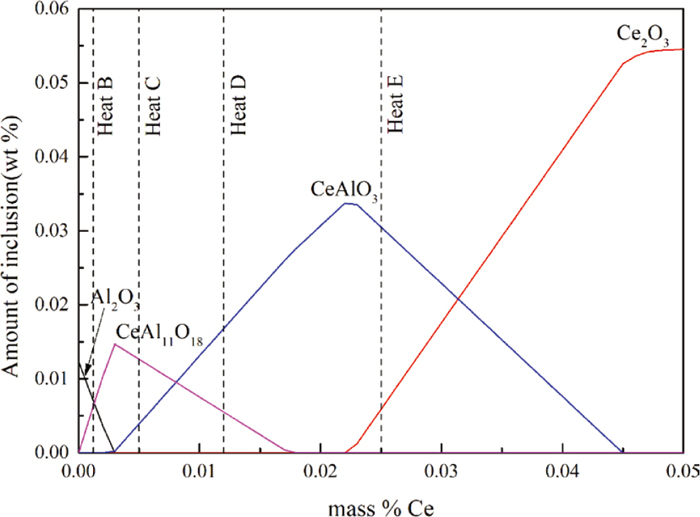
Evolution process of Ce on Al2O3 at 1873 K. (Online version in color.)
In heat A and B, the Ce content was less than 0.003 mass%. Under this condition, Al2O3 and CeAl11O18 were the stable inclusions in the molten steel. In heat A, only homogeneous Al2O3 and heterogeneous Al2O3 + CeAl11O18 inclusions were found. The types of inclusions were completely consistent with thermodynamic calculation results. The Ce content in heat B was higher than that in heat A, there were no homogeneous Al2O3 found in the molten steel. The oxide was mainly composed of heterogeneous Al2O3 + CeAl11O18 and homogeneous CeAl11O18. However, a small amount of CeAlO3 inclusions wrapped by CeAl11O18 were found in the sample of heat B. This may be because the local area of the melt reached the thermodynamic formation conditions of CeAlO3 immediately after Ce addition. CeAlO3 was only a transient product. As holding time goes by, CeAlO3 evolved into stable CeAl11O18 inclusions again. In the heat C and D, with the increase of the Ce content, thermodynamic calculation shows that the stable oxides are CeAl11O18 and CeAlO3. According to the analyzed results of SEM and EDS, it can be seen than experimental results were consistent with thermodynamic calculations results. In heat E, the Ce content of the molten steel reached 0.025 mass%. Under this condition, the oxides stably present in the molten steel were CeAlO3 and Ce2O3. After the addition of Ce, the oxides present in samples E2 and E3 were consistent with the results of thermodynamic calculations. Since Ce has a strong affinity for S in molten steel, a small amount of Ce2O3–CeS inclusions appeared in sample E2. However, in sample E3, Ce2O3–CeS was completely wrapped by CeAlO3, which indicated that the Ce2O3–CeS produced by the transient stage was gradually transformed into stable CeAlO3.
3.2.2. Formation Condition of Ti-containing OxidesIn order to study the effect of Al and Ti content on the formation of oxides, Factsage 7.2 software was used to calculate the stability phase diagram of Al–Ti–O in Fe-17Cr-9Ni steel. In the five heats after Al and Ti added, the compositions of the melt were marked in the phase diagram. The results were shown in Fig. 9. It can be seen that the compositions of sample 3 in five heats were located in the liquid oxide phase field. According to the previous studies,28,29) liquid Al2O3–TiOx inclusions obtained by modifying Al2O3 with Ti were found. Therefore, the Ti content was sufficient to modify Al2O3 into Al2O3–TiOx under thermodynamic conditions at the present work.
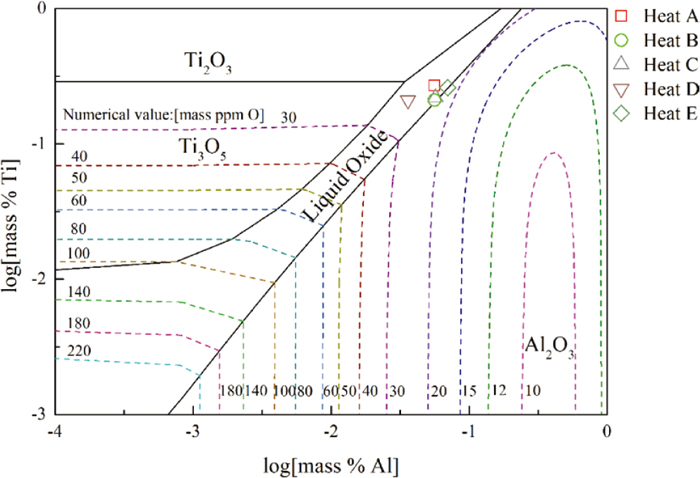
Calculated oxide stability diagram of Al–Ti–O system with iso-oxyen contours (in ppm) in Fe-17Cr-9Ni steel at 1873 K. (Online version in color.)
In our previous research,30) the equilibrium solubility products of TiN in Fe-17Cr-9Ni austenite stainless steel at 1873 K had been calculated. In this work, the contents of titanium and nitrogen of sample 3 are marked in Fig. 10. It can be seen that the five groups of heats had reached the thermodynamic conditions of TiN formation. Moreover, the nitrogen content corresponding to the dashed line in Fig. 10 was the nitrogen content in the raw material. When the nitrogen content in the original molten steel is 0.0123 mass%, as long as the titanium content exceeds 0.21 mass%, the TiN can be formed in the molten steel. Therefore, after the addition of titanium in the melts of five heats, TiN can be formed in the molten steel immediately.
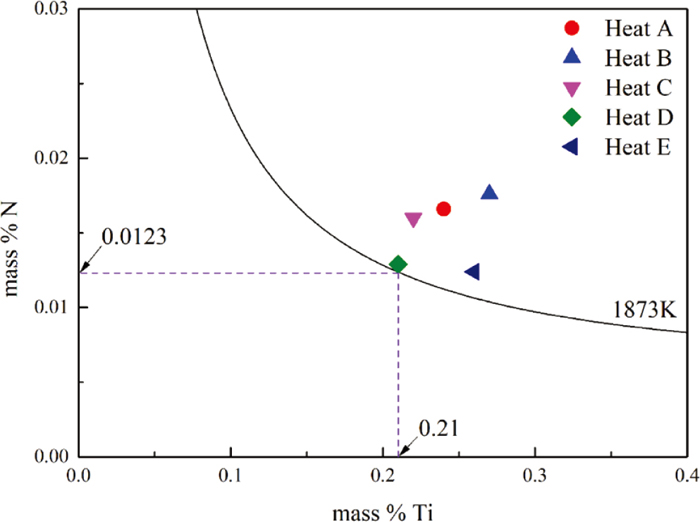
Equilibrium phase diagram of TiN in Fe-17Cr-9Ni austenite stainless steel at 1873 K. (Online version in color.)
In heat A, B and C, the Al, Ce and Ti were added to the molten steel in sequence, the evolution of inclusions in the molten steel with composition changes is shown in Fig. 11. After the addition of Al, the content of Ce in the inclusions increased with the increase in the content of Ce in the molten steel. The evolution of the oxides in the molten steel was: Al2O3 → Al2O3 + CeAl11O18 → CeAl11O18 → CeAl11O18 + CeAlO3 → CeAlO3. After the addition of Ti, the main inclusions appeared in the molten steel as TiN, and the newly formed TiN was easily precipitated with the oxide as the core. In terms of oxides, Ti can modify Al2O3 into Al2O3–TiOx complex oxide, but has no effect on the composition of Ce-containing oxides.
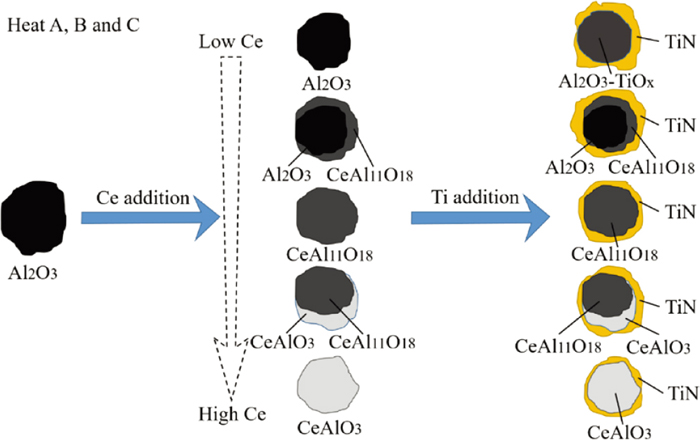
Schematic diagram of evolution process of inclusions in heat A, B and C. (Online version in color.)
In heat D and E, the order of adding alloy elements to the molten steel was: Al → Ti → Ce. Figure 12 shows the evolution process of inclusions in these two heats. After aluminum deoxidation, Al2O3 with different sizes appeared in the molten steel. After the addition of titanium, it took some time for the smaller size (less than 2 μm) solid phase Al2O3 to liquid phase Al2O3–TiOx inclusions. At the same time, Al2O3 could also be acted as the nucleation core of TiN. Due to the simultaneous effects of Ti on the modification of smaller size Al2O3 and precipitation of TiN, spherical Al2O3–TiOx inclusion that was completely wrapped by TiN formed. However, for larger size Al2O3, the modification process of Ti took more time, and the oxides could not be completely wrapped by TiN. Since it took sufficient time for the inclusions in the molten steel to reach thermodynamic equilibrium, the inclusions observed in this experiment were only in a transient stage. After Ce was added, the Al2O3 in the molten steel was completely modified to Ce-containing oxides due to the higher Ce content (relative to heat A, B and C). According to the level of Ce in the oxide, there are three types of Ce-containing oxides: CeAlO3 with CeAl11O18 core, pure CeAlO3 and Ce2O3. TiN inclusions appeared in the outer layers of the first two types of oxides, but no TiN inclusions were found in the outer layers of Ce2O3. According to previous studies,26) the lattice disregistry between Ce2O3 and TiN was higher than 12%, indicating Ce2O3 was not easy to be the nucleation core of TiN. Unlike the larger Al2O3 modified by Ce, the presence of TiN in the outer layer prevents Ce from modifying the oxide. The smaller size Al2O3–TiOx oxide completely wrapped by TiN had not changed after Ce added.
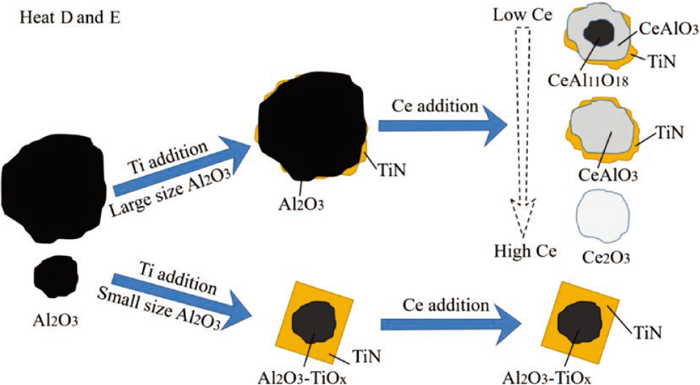
Schematic diagram of evolution process of inclusions in heat D and E. (Online version in color.)
In the current work, the formation and evolution process of inclusions after Al, Ce and Ti added in Fe-17Cr-9Ni stainless steel was studied. Combining experimental observation and thermodynamic calculation results, the evolution mechanism of inclusions was explained. The results were summarized as follows:
(1) Ce could effectively modify Al2O3 into Ce-containing oxides. As the Ce content in the molten steel increased, the inclusions gradually changed to Ce-rich oxides. The modification sequence of Ce on the Al2O3 inclusion was as follows: Al2O3 → CeAl11O18 → CeAlO3 → Ce2O3.
(2) The content of N in the original molten steel was 0.0123 mass%, the lowest Ti content when TiN formed in the molten steel at 1873 K was 0. 21 mass%. Therefore, after the addition of titanium in the melts of five heats, TiN could be formed in the molten steel immediately.
(3) When Al2O3–TiOx was completely wrapped by TiN. After adding Ce, TiN in the outer layer could prevent Ce from modifying the oxide in the inner layer.
The authors would like to express their appreciation to the State Key Laboratory of Advanced Metallurgy at University of Science and Technology Beijing for financial support, to Ruipu Technology Group Company for providing experimental raw materials.