2021 Volume 61 Issue 10 Pages 2654-2665
2021 Volume 61 Issue 10 Pages 2654-2665
The processes leading to hydrogen-related fracture in X80 pipeline steel with a stress concentration have been investigated comprehensively through observations of fracture surfaces and subsidiary cracks, stress analyses, crack initiation and propagation analyses and crystallographic analyses of fracture surfaces. Fracture morphology showed quasi-cleavage (QC) fracture under various amounts of hydrogen. It was found that QC cracks initiated in hydrogen-charged specimens in an area ranging from the notch tip to 100 µm inside based on interrupted tensile tests until just before fracture strength. A fracture surface topography analysis (FRASTA) revealed that QC cracks initiated at the notch tip. A finite element analysis indicated that the equivalent plastic strain was maximum at the crack initiation site at the notch tip. A backscattered electron image showed that nanovoids of 50–250 nm in diameter were present near the initiation site. Regarding the crack propagation process, field emission scanning electron microscopy (FE-SEM), electron backscattered diffraction (EBSD) and FRASTA results indicated that some microcracks in ferrite grains coalesced in a stepwise manner and propagated. Trace analyses using EBSD revealed that the QC fracture surface consisted of {011} slip planes, {001} cleavage planes and non-specific index planes. These findings indicate that QC fracture initiated at the notch tip due to the interaction between dislocations and hydrogen associated with local plastic deformation, and propagated in a stepwise manner by coalescence through vacancies, nanovoids and microcracks on various planes associated with/without plastic deformation in ferrite grains.
For the construction of a hydrogen energy-based society to achieve a low-carbon economy, it must be possible to transport hydrogen in large quantities at low cost using existing city natural gas pipelines. However, hydrogen embrittlement is a concern when liquefaction due to large earthquakes occurs, inducing large strain in pipelines caused by sideways floatation. One of the authors evaluated the hydrogen embrittlement susceptibility of smooth X80 pipeline steel specimens and reported that the formation of strain-induced vacancies was enhanced by hydrogen; consequently, fracture surfaces changed from typical dimples with nuclei of inclusions and precipitates into flat dimples without nuclei with increasing hydrogen content and decreasing strain rate.1) These changes presumably increased hydrogen embrittlement susceptibility. In an actual usage environment, it is more important to investigate the safety assessment and fracture mechanism of X80 steel with a stress concentration since corrosion pits and scratches may be present on the pipeline surface.
Intergranular (IG) fracture2,3) and quasi-cleavage (QC)4,5,6,7,8) fracture have been widely recognized as hydrogen embrittlement fracture morphologies in martensitic steel among representative high-strength steels. It has been reported that IG fracture is associated with a stress-controlled process because it occurs in the specimen interior away from the notch tip, corresponding to the maximum principal stress position in the tensile axis direction. While the QC fracture is associated with a strain-controlled process because it occurs at the notch tip corresponding to the maximum equivalent plastic strain position.9,10) However, the controlling factor of hydrogen embrittlement for X80 steel with a lower-strength, ferrite-bainite dual-phase microstructure may be different from that of a high-strength, single-phase martensitic microstructure. The initiation and propagation behavior of fractures may also differ between the two phases.
The main fracture morphology of low- and medium-strength ferrite matrix steels was reported to be QC fracture11,12,13) with shallow dimples.1,14) Regarding the initiation area of QC fracture, it was reported that nanovoids were observed beneath the QC fracture surface of X80 steel that fractured in the presence of hydrogen.11) Regarding the propagation area of QC fracture, it was reported that dislocation structure growth was suppressed by the presence of hydrogen and that QC cracks propagated along {001} cleavage planes in pure iron.12,13) However, few studies have examined the series of QC fracture processes from the incubation stage to crack initiation to crack propagation using identical material throughout the various analyses. As a result, the nature of QC fracture has not been clarified yet.
In the present study, the processes leading to hydrogen-related fracture in X80 pipeline steel with a stress concentration have been investigated comprehensively based on observations of fracture surfaces and subsidiary cracks, stress analyses, crack initiation and propagation analyses, and crystallographic analyses of fracture surfaces. Additionally, the common and different features of hydrogen-related QC fracture between the ferrite- bainite dual-phase microstructure and other single-phase microstructures were also investigated.
Specimens were made of X80 pipeline steel having the chemical composition shown in Table 1. Flat plate specimens of 170 mm in length along the direction of the tube axis were machined from actual pipelines. The specimens included a parallel part with a gage length of 20 mm, a width of 10 mm, and a thickness of 2.0 mm and were notched in the center of the gauge region as a stress concentration factor (Kt: 3.6) for tensile tests. Plate specimens with a length of 20 mm, a width of 10 mm, and a thickness of 2.0 mm were also machined for hydrogen analyses. The specimen surfaces were polished with #800 emery paper to keep the surface state uniform. Figure 2 shows the microstructure of an X80 specimen observed by FE-SEM. The dark gray area represents ferrite and the light gray area represents bainite. The proof stress was 580 MPa and tensile strength was 720 MPa.
| C | Si | Mn | P | S | Nb | Mo | Ti |
|---|---|---|---|---|---|---|---|
| 0.08 | 0.22 | 1.85 | 0.007 | 0.002 | 0.05 | 0.12 | 0.02 |

Geometry and dimensions of specimen for tensile test.

Microstructure of X80 pipeline steel observed by FE-SEM.
Specimens were electrochemically precharged with hydrogen. Hydrogen was charged in an aqueous solution of 0.1 N NaOH with 0 to 10 g·L−1 of NH4SCN at a temperature of 30°C with a current density of 5 A·m−2 until the hydrogen concentration at the surface and center of the specimens reached an equilibrium state. Hydrogen content was measured by thermal desorption analysis (TDA) using a gas chromatograph with a semiconductor gas sensor as the detector. TDA was conducted at a heating rate of 100°C· h–1 from 0 to 200°C.
2.3. Hydrogen Embrittlement SusceptibilityTensile tests were carried out at a crosshead speed (C.H.S.) of 0.005 to 1 mm·min−1 at 30°C with/without hydrogen charging. Hydrogen charging was conducted concurrently with the tensile tests for hydrogen-charged specimens after precharging.
2.4. Determination of Hydrogen-related Crack Initiation SiteThe controlling factor of the hydrogen-related crack initiation site was determined both experimentally and by stress analysis. Specimens were electrochemically precharged with hydrogen in an aqueous solution of 0.1 N NaOH containing 0.1 g·L−1 of NH4SCN at 30°C with a current density of 5 A·m−2 for 72 h, and loading was applied during hydrogen charging. The specimens were unloaded immediately at 650 MPa, 100 MPa lower than the maximum fracture strength. After unloading, the specimens were kept in a thermostatic chamber at 30°C for 144 h for degassing; subsequently, their hydrogen content was analyzed by TDA and confirmed to be 0 ppm. After that, degassing was performed under this condition. Following degassing, tensile tests were conducted in air until failure, and the hydrogen-related crack initiation site of the fracture surface was determined through observation by FE-SEM.
An elastic-plastic stress analysis was performed using ABAQUS finite element analysis software and an axisymmetric 1/8 model. The minimum element size in the analysis model was 0.01 mm and the total number of elements was 40000. The principal stress and the equivalent plastic strain distribution in the vicinity of the notch tip were determined at applied stress of 650 MPa in the tensile test. The controlling factor of the hydrogen-related crack initiation site of X80 steel was investigated by comparing the initiation sites obtained experimentally with the stress and strain distributions obtained analytically.
2.5. Hydrogen-related Crack Initiation Site and Propagation Behavior Analyzed by FRASTAHydrogen-related crack initiation and propagation behavior was analyzed using FRASTA15) which makes it possible to computationally reconstruct the fracture processes from three-dimensional images of conjugate areas in opposing fracture surfaces. Specimens were precharged with hydrogen under the same conditions as in section 2.4, followed by charging with hydrogen concurrently during the tensile tests. The obtained fracture surfaces were observed by FE-SEM. Fracture surface images were taken from three directions by tilting the sample stage to −5°, 0°, and +5°. Three-dimensional images of the fracture surfaces were then constructed using MEX software. The hydrogen-related crack initiation point was determined, and propagation behavior was also analyzed by superimposing the two conjugate topographic maps until there was no gap between them, and then gradually increasing the relative distance between the two surfaces in the tensile axis direction.
2.6. Crystallographic Analysis of Hydrogen-related Fracture SurfaceTwo-surface trace analyses were carried out using EBSD to clarify hydrogen-related fracture initiation and propagation behavior in X80 steel. Specimens were precharged with hydrogen under the same conditions as in section 2.4, followed by charging with hydrogen concurrently during the tensile tests. The fracture specimen was cut at a 5-mm distance from the fracture surface. A Ni layer was electrodeposited onto the fracture surface with an aqueous solution of 150 g·L−1 Ni2SO4 + 15 g·L−1 NH4Cl + 15 g·L−1 H3BO4 at a current density of 200 A·m−2 at room temperature for 3 h while stirring with a stirring bar to protect the surface. One side perpendicular to the fracture surface was mechanically polished with emery papers of #800, #1000, and #2000, and then with diamond suspensions of 9 μm and 3 μm in diameter, after that with colloidal silica of 0.05 μm in diameter, and finally cleaned with methanol. The crystallographic features of the hydrogen-related fracture propagation area were analyzed by EBSD measurements. The acceleration voltage was at 15 kV and the beam step size was 0.05 μm.
The data obtained were converted into inverse pole figure (IPF) maps by orientation imaging microscopy (OIM) analysis, and the traces of the {011} and {001} planes to arbitrary pixels beneath the fracture surface were displayed, and then the trace analyses were conducted. The specimen surfaces were then indented at two arbitrary points with a Vickers indenter, followed by polishing with colloidal silica of 0.05 μm in diameter, and subsequently the trace analyses were performed again. After that, SEM images were taken of the analyzed area. The actual translation distances between the first and second trace planes were measured by superposing the FE-SEM images of the first and second planes. The points consistent with the calculated translation distance Δx obtained with Eq. (1) were regarded to be parallel to the trace plane.
| (1) |
| (2) |
The area near the fracture surface in the center region having a large plastic constraint in the specimen thickness direction was observed using FE-SEM by polishing the plane from 2 mm to 1 mm in thickness perpendicular to the hydrogen-related fracture surface electrodeposited with the Ni layer. EBSD analyses were also conducted at the tips of subsidiary cracks near the initiation point of the hydrogen-related fracture.
The charging time was varied to determine how long it took to reach an equilibrium hydrogen concentration at both the surface and center of the specimens. Hydrogen desorption profiles of specimens charged with hydrogen in a 0.1 N NaOH solution containing 5 g·L−1 of NH4SCN at a current density of 5 A·m−2 at 30°C for various charging times are shown in Fig. 3(a) as an example. The hydrogen content is shown as a function of the charging time in Fig. 3(b). It reached almost a constant value after approximately 72 h, which was determined to be the charging time required to obtain an equilibrium hydrogen concentration at both the surface and center of the specimens.

(a) Thermal desorption profiles and (b) hydrogen content of specimens charged by cathodic electrolysis in a 0.1 N NaOH solution containing 5 g·L−1 of NH4SCN at a current density of 5 A·m−2 for various hydrogen charging times.
Figure 4 shows thermal desorption profiles and hydrogen contents of specimens charged with hydrogen in a 0.1 N NaOH solution containing 0 to 10 g·L−1 of NH4SCN at a current density of 5 A·m−2 at 30°C for 72 h. The hydrogen contents of specimens charged in solutions containing NH4SCN of 0, 0.1, 1, 5, and 10 g·L−1 were 0.06, 0.34, 0.38, 0.53, and 1.24 mass ppm (expressed as ppm in the following), respectively.

Thermal desorption profiles and hydrogen contents of specimens charged by cathodic electrolysis in a 0.1 N NaOH solution containing various amounts of NH4SCN at a current density of 5 A·m−2 for 72 h.
Figure 5 shows nominal stress-displacement curves of uncharged and hydrogen-charged specimens containing initial hydrogen amounts of 0.06, 0.34, 0.38, 0.53, and 1.24 ppm obtained at a crosshead speed of 0.05 mm·min−1. Nominal stress on the vertical axis was defined as the load value divided by the net notched area. For uncharged specimens, the tensile strength of the notched specimens was higher than the 720 MPa recorded for the smooth specimens because of the plastic deformation constraint near the notch. The maximum fracture strength and elongation decreased with increasing hydrogen content. Figure 6 shows nominal stress-displacement curves obtained at various crosshead speeds of 0.005, 0.05, 0.5, and 1.0 mm·min−1 for uncharged and hydrogen-charged specimens containing an initial hydrogen amount of 0.38 ppm. The stress-displacement curve of a specimen at a C.H.S of 0.05 mm·min−1 is shown as a representative result due to less dependence of the curve on C.H.S. for uncharged specimens. The maximum fracture strength and elongation decreased with decreasing C.H.S. These results indicate that hydrogen embrittlement susceptibility rose with increasing hydrogen content and decreasing C.H.S.

Nominal stress-displacement curves of uncharged and hydrogen-charged specimens containing various initial amounts of hydrogen at a crosshead speed of 0.05 mm·min−1. (Online version in color.)

Nominal stress-displacement curves of uncharged and hydrogen-charged specimens containing 0.38 ppm of hydrogen under various crosshead speeds. (Online version in color.)
Figure 7(a) shows a whole image of the fracture surface of an uncharged specimen, (b) shows a magnified image near the notch tip indicated in the green rectangle in image (a), (c) shows a whole image of hydrogen-charged specimens containing an initial hydrogen content of 0.34 ppm, and (d) shows a magnified image near the notch tip indicated in the red rectangle in image (c). The uncharged fracture specimen shows a large reduction in area and the specimen surface shows microvoid coalescence (MVC) over the whole area, representing typical ductile fractography. In contrast, the hydrogen-charged fracture specimen shows less reduction in area than the uncharged one and the specimen surface exhibits QC, representing typical hydrogen embrittlement fractography at both notch tip areas and near a part of the side surface areas. However, IG fracture was not observed.

FE-SEM images of fracture surfaces of (a) uncharged specimen, (b) magnified image near notch tip area indicated in the green rectangle in image (a), (c) hydrogen-charged specimens, and (d) magnified image near notch tip area indicated in the red rectangle in image (c). (Online version in color.)
Figure 8(a) shows stress-displacement curves obtained for identifying the hydrogen-related crack initiation site. A specimen containing an initial hydrogen content of 0.34 ppm was preloaded up to 650 MPa, 100 MPa lower than fracture strength, as indicated by the red line, and unloaded as indicated by the blue dotted array, followed by degassing and reloading until fracture in air as indicated by the black line. Figure 8(b) shows an FE-SEM image of the fracture surface near the notch tip. Although almost all areas were MVC owing to degassing of hydrogen, a QC crack was observed in a small area ranging from the notch tip to 100 μm inside as indicated in the yellow dashed rectangle. This observation reveals that QC cracks initiated in the area ranging from the notch tip to 100 μm inside. However, these interrupted tensile tests did not make it possible to narrow the QC crack initiation site down to only the area ranging from the notch tip to 100 μm inside. Therefore, FRASTA was used in an attempt to pinpoint the site as explained below.

(a) Stress-displacement curves for identifying the crack initiation site; application of preloading up to 650 MPa just before fracture strength with hydrogen and subsequent unloading, degassing and reloading until fracture; (b) FE-SEM image of fracture surface at the notch tip. (Online version in color.)
The results analyzed by FRASTA on the basis of three-dimensional images of opposing fracture surfaces of specimens containing an initial hydrogen content of 0.34 ppm are shown in Fig. 9. The d values in the upper right corner of each figure indicate the distance between the two images. The blue area represents the overlapping of the opposing fracture surfaces, and the green area represents no overlap. As the relative distance was gradually increased from (a), the completely overlapped stage, a sequence of gaps first formed in a portion of the notch tip as shown in (c), after which the gaps spread out from the whole notch tip area to the right toward the specimen interior as shown in (d)–(i). This means that a QC crack initiated at the notch tip and then propagated to the specimen interior. Although the interrupted tensile tests did not make it possible to narrow the QC crack initiation site down to only the area ranging from the notch tip to 100 μm inside, FRASTA made it possible to determine that the crack initiation site was the notch tip.

Fracture initiation and propagation process of hydrogen-charged specimens analyzed by FRASTA. (Online version in color.)
Figure 10 shows the distribution of the principal stress and equivalent plastic strain along the tensile axis near the notch tip as analyzed by FEM. The principal stress was maximum at approximately 130 μm inside from the notch tip and the equivalent plastic strain was maximum at the notch tip. On the basis of the interrupted tensile test and FRASTA results, the fracture initiation site was determined to be the maximum equivalent plastic strain point at the notch tip and not the maximum principal stress point at approximately 130 μm inside from the notch tip.

Distribution of principal stress and equivalent plastic strain at the notch tip analyzed by FEM. (Online version in color.)
Figure 11(a) shows a schematic diagram of a cross section near the hydrogen-related fracture of a specimen containing initial hydrogen of 0.34 ppm and (b) shows an FE-SEM image near the intersection area of the notch tip and the QC fracture surface as indicated by the green dashed line in Fig. 11(a). A subsidiary crack as well as the main crack leading to the final fracture surface can be observed. Figure 11(c) shows a magnified backscattered electron (BSE) image of the crack initiation area indicated by the blue dashed line in Fig. 11(b). A number of nanovoids of 50–250 nm in diameter were observed near the QC crack initiation site, but no nanovoids were observed in other areas except near the notch tip.

(a) Schematic diagram of a cross section near the hydrogen-related fracture, (b) FE-SEM image near the intersection area of notch and QC fracture surface indicated in the green rectangle in image (a), and (c) magnified BSE image at crack initiation area indicated in the blue rectangle in image (b). (Online version in color.)
Figure 12 shows a magnified FE-SEM image of a subsidiary crack tip area observed near the QC crack initiation site as indicated in the red dashed rectangle in Fig. 11(b). A coalesced stepwise pattern with some microcracks of 1–3 μm in length was observed at the subsidiary crack tip. A nanovoid or blunt microcrack of 0.5 μm in diameter was also observed away from the subsidiary crack tip.

FE-SEM image of subsidiary crack tip area near the notch and QC fracture surface indicated in the red rectangle in Fig. 11(b). (Online version in color.)
Figure 13 shows (a) IQ, (b) KAM, and (c) IPF maps near the subsidiary crack tip close to the notch as observed in Fig. 11(b). Figure 13(d) shows a magnified IQ map as indicated in the red dashed rectangle in Fig. 13(a). The bainite phase with larger lattice disorder had a lower CI value and a higher KAM value than the ferrite phase. This means that the darker areas in the IQ map and with a higher value in the KAM map are attributed to the bainite phase. These results in (a) to (d) reveal that the subsidiary crack propagated not along the grain boundaries but in the ferrite grains and ran across the ferrite grain boundaries.

(a) IQ, (b) KAM and (c) IPF maps near the subsidiary crack tip, and (d) magnified IQ map near the subsidiary crack tip of a hydrogen-charged specimen analyzed by EBSD. (Online version in color.)
Figure 14 shows cross-sectional views perpendicular to the tensile axis in the QC crack propagation area at approximately 200 μm inside from the notch tip of a hydrogen-charged specimen analyzed by FRASTA. This analyzed area is shown as a yellow rectangle in Fig. 9(a). The d values in the upper right corner of each figure indicate the distance between the two fracture surfaces. The crack propagated from the left side as shown in (b) and appeared to propagate successively toward the right as shown in (c) to (h). However, the crack did not always propagate successively and microcracks of 1 to 2 μm in length formed at the front away from the main crack tip; the main crack and the microcracks then coalesced in a stepwise manner. These FRASTA results are in excellent agreement with the direct observation of the subsidiary crack propagation behavior obtained by FE-SEM in Fig. 12.

Cross-sectional views of crack tip showing successive stages of QC fracture crack propagation of a hydrogen-charged specimen analyzed by FRASTA. (Online version in color.)
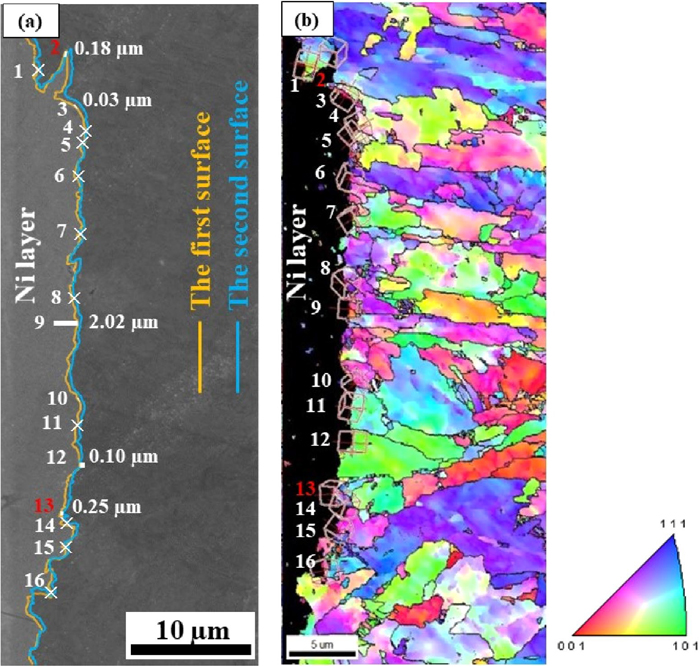
Figure 15 shows a trace analysis of the {011} plane of the crack propagation area at approximately 0.14 to 0.21 mm inside from the QC initiation point; (a) shows superimposed SEM images of the first and second surfaces, and (b) presents the corresponding IPF map. The boundary between the nickel plating layer and the fracture surface on the first plane is represented by an orange line and that on the second plane is represented by a blue line. The calculated boundary translation distances between the first and second trace planes are expressed as white bars. The analyzed points, at which the calculated boundary distances parallel to the {011} trace were consistent with the actual boundary distances, are indicated by red numbers. Three of the 16 points, denoted as Nos. 1, 5, and 15, were parallel to the {011} trace for a ratio 3/16. Figure 16 shows a trace analysis of the {001} plane in the same area as in Fig. 15; (a) shows superimposed SEM images of the first and second surfaces, and (b) presents the corresponding IPF map. Two of the 16 points, denoted as Nos. 2 and 15, were parallel to the {001} plane for a ratio of 2/16.

Trace analysis of the {011} plane of a cross section near QC fracture surface; (a) FE-SEM images of the first and second surfaces, and (b) EBSD orientation map. (Online version in color.)
Trace analysis of the {001} plane of a cross section near QC fracture surface; (a) FE-SEM image of the first and second surfaces, and (b) EBSD orientation map. (Online version in color.)
Figure 17 shows the IPF maps of (a), (b) and (c), and KAM maps of (f), (g) and (h) at the No. 1, 5, and 15 points along the {011} trace in Fig. 15, and the IPF maps of (d) and (e), and KAM maps of (i) and (j) at the No. 2 and 13 points along the {001} trace in Fig. 16, respectively. Average KAM values along the {011} trace were 0.68 to1.85, and those along the {001} trace were 0.44 and 0.47. The KAM values near fracture surface points along the {011} plane tended to be higher than those along the {001} plane. These EBSD results reveal that the QC fracture surface in the propagated area at 0.14 to 0.21 mm in front from the crack initiation site of the X80 specimens consisted of the {011} slip plane, the {001} cleavage plane of the bcc lattice and non-specific index planes.

IPF maps of (a), (b) and (c), and KAM maps of (f), (g) and (h) near fracture surfaces corresponding to the {011} trace. IPF maps of (d) and (e), and KAM maps of (i) and (j) near fracture surfaces corresponding to the {001} trace. Average KAM values are also shown in the lower row. (Online version in color.)
The incubation stage prior to crack initiation is discussed first among the processes leading to QC fracture of hydrogen-charged X80 specimens. Observation of fracture surfaces obtained in the interrupted tensile tests just before fracture strength as indicated in Fig. 8 made it possible to narrow the QC crack initiation site down to the area ranging from the notch tip to 100 μm inside, and the results obtained by FRASTA in Fig. 9 revealed that the QC crack initiation site was at the notch tip. Crack initiation was presumably associated with strain-controlled, rather than stress-controlled, fracture because the crack tip point corresponded to the maximum equivalent plastic strain point, not the maximum principal stress point, as shown in Fig. 10. Sudden fracture occurs upon reaching a critical stress level in the case of stress-controlled fracture, whereas dislocation slips are increasingly activated in plastic strain in the case of strain-controlled fracture. It is inferred that the dislocation density and vacancy concentration increase at the crack tip prior to crack initiation in the incubation stage. Fracture often occurs in the elastic region of the load-displacement curve as the load increases for notched high-strength steels charged with hydrogen.16,17) In contrast, the results for the X80 specimens showed that they did not fracture suddenly but rather under plastic deformation as the load was increased as indicated in Figs. 5 and 6. This result supports the idea that fracture is not attributed to sudden decohesion of atomic bonding but to the accumulation of lattice defects and crack initiation and propagation.
First, the lattice defect density at the crack tip in the incubation stage prior to crack initiation is discussed here. The equivalent plastic strain at the crack tip was approximately 17% under applied stress of 650 MPa at which QC fracture was initiated as shown in the stress analysis results in Fig. 10. Fracture occurred in the apparent elastic region of the stress-displacement curve, but these results indicate that large plastic deformation occurred locally at the crack tip. Presumably, dislocation slips were activated, the dislocation density increased, and the dislocations acted as hydrogen trapping sites at the crack tip prior to crack initiation. Vacancies also formed by a combination of edge dislocations with opposite characteristics located on slip planes at an atomic plane distance apart18) and the cutting of screw dislocations.19,20) Stress-induced diffusion of hydrogen also occurred. These three factors probably caused the notably high hydrogen concentration at the crack tip.
A number of nanovoids of 50–250 nm in diameter were observed near the QC crack initiation site as indicated in Fig. 11(c). One of the authors reported that an X80 pipeline steel specimen subjected to 8% strain in the presence of hydrogen displayed a greater amount of vacancy defects than a specimen subjected to the same strain in the absence of hydrogen as evaluated by the hydrogen tracer method.1) The equivalent plastic strain reached approximately 17% at the crack tip of a notched specimen subjected to only stress of approximately 650 MPa in an apparent elastic region, although large plastic strain was needed for a smooth specimen. The high strain field presumably led to the formation of strain-induced vacancies and the presence of hydrogen enhanced that formation. The high concentration and accumulation of vacancies prior to reaching a stress level of 650 MPa initiated QC fracture, and these processes corresponded to the incubation stage.
Next, the process from the incubation stage to crack initiation is discussed. It is assumed that ductile fracture tended to occur in the local area owing to a higher hydrogen concentration and a higher density of vacancy defects as applied stress was increased. Subsequently, nanovoids probably formed in the soft ferrite grains as shown in Fig. 11(c) because vacancy defects stabilized by hydrogen acted as nuclei, coalesced and grew.14,21,22) A study based on a molecular dynamics simulation reported that complexes of hydrogen vacancies (VaHn) formed and VaHn grown by plastic strain acted as nuclei of nanovoids.23) Another conceivable possibility is that nanovoid formation and growth were enhanced due to the triaxial stress state at the notch tip. The notch tip where nanovoids were observed corresponded to the initiation site of QC fracture.
These results and observations indicate that vacancy defects formed by dislocation cutting in soft ferrite grains were stabilized by a high hydrogen concentration, densified and accumulated in processes corresponding to the incubation stage. Subsequently, these densified and accumulated vacancy defects presumably acted as nuclei, coalesced and grew, resulting in QC crack initiation because of plastic instability.24) It is inferred that no IG fracture resulted from these processes in X80 steel with a soft ferrite phase unlike that seen for high-strength steels. Accordingly, QC fracture associated with local plastic deformation occurred at a lower stress level than the stress that causes cracks due to grain boundary decohesion.
4.2. QC Crack Propagation ProcessThe propagation process after QC crack initiation in hydrogen-charged X80 specimens is discussed. A nanovoid or blunt microcrack of approximately 0.5 μm in diameter was observed in front of and away from the subsidiary crack tip near the QC crack initiation area. This suggests that the nanovoid or blunt microcrack formed and grew at the maximum triaxial stress point in front of and away from the crack tip. The subsidiary crack consisted of coalesced microcracks of 1–3 μm in length. This QC crack propagation behavior was also observed in the FRASTA results exhibiting a coalesced stepwise pattern with some microcracks as shown in Fig. 14. QC crack propagation due to the coalescing of microcracks and microvoids was also reported for ASTM A490 high-strength bolt steel,25) S10C steel,26) D6ac steel27) and single crystal of pure iron.28) The QC fracture surface consisted of several facets of approximately 1 to 5 μm in length surrounded by fragmentary tear ridges as indicated in Fig. 7(d). This observation enables us to consider that tear ridges (denoted by white lines) similar to torn traces appeared around facets on the fracture surface. The EBSD results in Fig. 13 revealed that the subsidiary crack propagated not along the phase boundaries but in the ferrite grains. Since local plastic deformation occurred more in the softer ferrite phase than in the bainite phase as the crack propagated, nanovoids or cracks formed and grew at the maximum triaxial stress point in front of and away from the crack tip. It is inferred that the QC crack propagated in this manner.
Next, crack propagation in the ferrite phase is discussed crystallographically. Two-surface trace analyses revealed that the propagation surface of the QC fracture consisted of the {001} cleavage fracture plane, the {011} slip plane of the bcc lattice and non-specific index planes. A possible reason that can be considered for fracturing on the {001} cleavage fracture plane in a part of the fracture surfaces is decohesion by hydrogen29,30) or suppression of dislocation slips by hydrogen31) concentrated at the crack tip. An increase in yield point and a decrease in elongation, i.e., suppression of dislocation slips, were reported for FeAl, AISI 304, 316, and 347 steels with an increasing vacancy concentration.32,33,34) Taking into account hydrogen trapping at vacancies, another possible reason that can be considered is a greater suppression of dislocation slips by the formation of hydrogen-vacancy complexes.
A possible reason for fracturing on not only the {011} slip plane but also on non-specific index planes is presumably that fracture occurred on the various planes as an axis of the [111] slip direction since the bcc lattice has no outstanding close-packed plane unlike a face-centered cubic (fcc) lattice and a tendency for cross-slips to occur is a characteristic of the bcc lattice. Another possible reason is that the plane of the first microcrack to occur differs from that of the connecting plane on which two fracture surfaces finally rupture since interconnected microcracks were observed at the subsidiary crack tip near the QC crack initiation site as described earlier. It was reported for martensitic steel that a QC crack propagated and fractured along the {011} slip plane5) and along the lath boundaries parallel to the {011} plane.6) It was also reported that a QC crack propagated along the {001} cleavage plane for pure iron.12,13) The QC cracks in the X80 steel used in the present study propagated not along a specific plane but along various planes unlike the results of previous studies. This is probably because X80 steel consists of a ferrite-bainite dual phase and fine grains rather than a single phase of ferrite. Points in common with previous reports for QC cracks in single phases of ferrite and martensite are that QC cracks propagated in the grains, propagated with plastic strain, and propagated in a stepwise manner by coalescence. One difference is that the QC fracture surface consisted of various planes, not a single crystallographic plane.
These findings obtained through various analyses for the processes leading to hydrogen-related QC fracture revealed that crack initiation corresponded to a strain-controlled process associated with plastic deformation at the notch tip, while crack propagation corresponded to mixed mechanisms of a stress-controlled process associated with less plastic deformation, as indicated by fracture on the cleavage plane, and a strain-controlled process associated with large plastic deformation, as indicated by fracture on the slip plane.
The processes leading to hydrogen-related fracture in X80 pipeline steel with a stress concentration have been investigated comprehensively based on various analyses. The findings obtained can be summarized as follows.
(1) As for crack initiation, hydrogen-charged specimens were unloaded just below fracture strength, followed by degassing and then reloaded until fracture in air. As a result, the QC crack area was observed in a range from the notch tip to 100 μm inside. FRASTA results indicated that QC cracks initiated at the notch tip. Stress analyses near the notch tip of hydrogen-charged specimens showed that the principal stress was maximum at approximately 130 μm inside away from the notch tip and that the equivalent plastic strain was maximum at the notch tip. A number of nanovoids of 50–250 nm in diameter were observed in the notch tip area (crack initiation site) beneath the QC fracture surface.
(2) As for crack propagation, a subsidiary crack observed near the QC crack initiation site propagated in the ferrite grains and consisted of a coalesced stepwise pattern with some microcracks of 1–3 μm in length. A nanovoid or blunt microcrack of 0.5 μm in diameter was also observed in front of the subsidiary crack tip. Therefore, the QC crack propagation behavior, in which microcracks do not always propagate successively but coalesce in a stepwise manner, was in excellent agreement with that seen in the FRASTA results.
(3) Two-surface trace analyses revealed that the QC fracture propagation surface consisted of the {001} cleavage fracture plane, the {011} slip plane of the bcc lattice and non-specific index planes. The KAM values near the fracture surface points along the {001} plane tended to be lower than those along the {011} plane.
(4) Considering the processes leading to QC fracture based on the various analysis results, the process in which vacancy defects formed by dislocation cutting in the soft ferrite grains were stabilized by the high hydrogen concentration, densified and accumulated corresponded to the incubation stage. Subsequently, these densified and accumulated vacancy defects presumably acted as nuclei, coalesced, grew, and resulted in crack initiation because of plastic instability. Finally, microcracks coalesced in a stepwise manner in the ferrite grains, resulting in crack propagation on various crystallographic planes associated with less to more plasticity. It is concluded that these processes correspond to the nature of QC fracture.