2021 Volume 61 Issue 4 Pages 1300-1308
2021 Volume 61 Issue 4 Pages 1300-1308
In this study, the decarburization behavior, and its effect on mechanical properties of 2000 MPa class martensitic steel were investigated with the purpose of collecting the basic data to realize the further strengthening of structural members for automobiles. Concerning the decarburization behavior, the maximum of the decarburization rate of 2000 MPa class martensitic steel with a basic chemical composition of 0.35%C-1.2%Mn was found around 750°C and the thickness of the decarburized zone grew proportional to the square root of time. The addition of Nb reduced the decarburization rate.
Concerning the effect of decarburization on mechanical properties, the decarburization significantly improved bendability even though the thickness of the decarburized layer is relatively thin. An observation of the crack behavior revealed that the initiation and propagation of the crack were suppressed by the decarburization layer. The resistance to delayed fracture was improved by decarburization. This improvement is presumed to be based on a similar mechanism for the improvement of bendability.
For long time, strengthening automotive steels has been proceeded for lightening automobiles and for improving crashworthiness. In recent years, ultrahigh strengthening of automotive structural parts has been performed not only from developing new steels but also combined with the ingenuity of the forming method, which is called the solution for ultrahigh strengthening.1) The ingenuity of the forming methods is, for example, hot stamping2) and bending based forming of ultrahigh strength cold rolled steel sheets.3)
The major obstacles to the practical application of ultrahigh strength steel are the deterioration of bendability and an increase in delayed fracture susceptibility. The deterioration of bendability not only makes it difficult to form ultrahigh strength cold rolled steel sheets, but also causes fracture without sufficient deformation of the members at the time of the collision, so that expected high absorbed energy is not obtained.
On the other hand, delayed fracture of automotive structural parts must be avoided from the viewpoint of safety. Many studies have been conducted on the delayed fracture susceptibility of automobile structural parts, and the susceptibility to delayed fracture of cold rolled steel sheets has been reported to increase when the tensile strength is 1200 MPa or more. In particular, it has been reported that the susceptibility to delayed fracture increases if a steel sheet contains a large amount of deformation induced martensite from retained austenite.4)
Hot stamped parts are formed in the austenite temperature region and quenched in the dies. Therefore, the residual tensile stress is much lower than that in cold stamped ultrahigh strength parts. Consequently, the susceptibility to delayed fracture of hot stamped parts is lower than that of cold stamped ultrahigh strength parts at the same strength level so that hydrogen induced delayed fracture has not been a critical issue for 1500 MPa class hot stamped parts. Since an increase in the strength to 2000 MPa of hot stamped parts decreases the resistance to delayed fracture significantly, a number of studies have been conducted to improve its susceptibility by proper alloy design and controlling microstructures. Concerning the microstructural control, it has been reported that grain refinement of prior austenite and the increase in the amount of retained austenite lower the susceptibility to delayed fracture.5,6,7) Concerning the alloy design, decreasing C and Mn and adding micro-alloy elements such as Nb, Ti, Mo have been reported to increase the resistance to delayed fracture.8,9,10,11,12,13,14,15)
In a conventional hot stamping process, steel sheets are heated to around 900°C and held for about 5 min in a furnace. In the usual case, nitrogen gas with a low dew point as a furnace atmosphere is used in order to avoid scale formation and surface decarburization as much as possible.
On the other hand, it has recently been reported that decarburization significantly improves bendability.16,17,18) However, concerning hot stamped parts, there are no reports on details of decarburization behavior and the relationship between bendability and thickness of the decarburized layer. In addition, no report was found regarding the effect of decarburization on the delayed fracture susceptibility of martensitic steel.
Therefore, we have investigated the decarburization behavior under the conditions similar to hot stamping heating and the effect of decarburization on bendability and susceptibility to delayed fracture of martensitic steels.
The chemical composition of the steels used in the experiment is given in Table 1. These steels are designed for producing 2000 MPa class hot stamped automotive parts. Steel D2 contains 0.05% Nb to investigate the effect of Nb on the behavior of decarburization and mechanical properties. The steels were melted in a vacuum furnace and cast into a 50 kg ingot. The ingot was reheated at 1250°C for 60 minutes and hot rolled to a 3 mm thick hot band at a finishing temperature of around 930°C. The hot band was directly put into an electric furnace of 600°C and kept for 60 minutes and cooled to the room temperature in the off-switched furnace. The microstructure of the hot bands consists of ferrite/pearlite. After pickling, the hot bands were cold rolled to 1.0 mm or 1.4 mm thick sheets.
| Steel | C | Si | Mn | P | S | Al | Nb | Ti | B | N |
|---|---|---|---|---|---|---|---|---|---|---|
| D1 | 0.35 | 0.11 | 1.2 | 0.0006 | 0.0007 | 0.04 | 0 | 0.01 | 0.001 | 0.0006 |
| D2 | 0.35 | 0.11 | 1.2 | 0.0007 | 0.0007 | 0.04 | 0.05 | 0.01 | 0.001 | 0.0007 |
The heat treatment was conducted using a direct current heating device or an electric furnace. In the case of the direct current heating, the samples of 1.0 mm or 1.4 mm in thickness were heated at 10°C/s to various temperatures for the preset time in the air and immediately quenched by water spray. The samples without decarburized zone were produced by machining the 1.4 mm sheet to 1.0 mm in thickness.
Specimens for delayed fracture tests were first machined (by electrical discharge machining) to a given profile from the cold rolled sheet and heat treated using an electric furnace in controlled atmospheres.
All samples were tempered at 170°C for 20 min to simulate BH-treatment before mechanical tests.
2.3. Microstructural Observations and Micro-hardness MeasurementsObservations of microstructure were carried out using optical microscopy (OM) and secondary electron microscopy (SEM). Micro-texture data obtained by electron back-scattering pattern (EBSP) analysis were used to determine the grain size of prior austenite.
The hardness was measured on a Micro-Vickers with an indentation load of 490 N.
2.4. Mechanical TestsThe tensile test was performed using samples according to the Japanese standard JIS 13B and a universal tensile test machine, AG-50kNXplus, Shimazu Ltd.
Bending tests were performed using a facility described in VDA 238-100. The critical bending angle was defined as the angle at which the bending load dropped by 30 N from the maximum value. The angle α shown in Fig. 1 was measured from the video-recorded pictures.

Schematic depiction of bending test. (Online version in color.)
The facility and the sample used for the delayed fracture test are shown in Fig. 2. The delayed fracture test was carried out in the way that the test sample was immersed in 10% ammonium thiocyanate solution under a maximum stress of 1300 MPa at the bottom of the notch, and the time to fracture was measured.

Testing machine and size of the specimen for delayed fracture tests. (Online version in color.)
To measure the total amount of accumulated hydrogen in the sample, the sample was immersed in a 10% ammonium thiocyanate solution for 1 h, 3 h, and 48 h. The accumulated hydrogen was determined by thermal desorption analysis (TDA) using a gas chromatograph (JTF-20AH), J-Science. The heating rate was 200°C/h.
To investigate the influence of heating temperature and time on the decarburization rate of Steel D1 and D2, we varied the decarburization temperature from 740°C to 800°C with an interval of 20°C, and the decarburization time for 5 and 15 minutes. Figure 3 shows two representative decarburized microstructures in the vicinity of the surface. The samples are D1 heated at 740°C and 760°C for 15 minutes in air. The decarburized zone is clearly distinguished. In addition, it can be seen that the sample heated at 740°C has some fine ferrite in the martensitic matrix whereas the microstructure of the sample heated at 760°C is a martensitic single-phase.

Representative decarburized microstructures in the vicinity of the surface. [The samples are Steel D1 heated at 740°C (a) (aligned fine grains indicated by arrows are ferrite) and 760°C (b) for 15 minutes in air.] (Online version in color.)
Figure 4 shows the thickness of the decarburized zone (TDZ) of Steel D1 heated in various conditions. The TDZ increases with decreasing temperature but the difference in the TDZ of the sample heated at 760°C from that of the sample heated at 740°C is slight so that the maximum of the decarburization rate is implied to lie near these temperatures. To clarify the time dependency of the TDZ, we decarburized Steel D1 at 790°C for various holding time. The result was shown in Fig. 5. The TDZ grew approximately proportionally to the square root of time.

Influence of decarburized temperature and time on the thickness of decarburized zone of Steel D1. (Online version in color.)
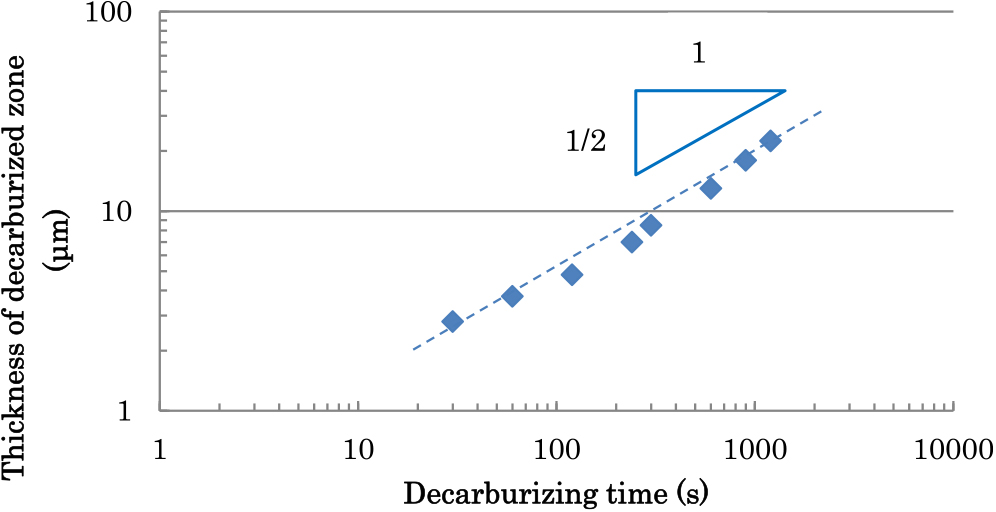
Relationship between decarburized time and thickness of decarburized zone (Steel D1, decarburized temperature: 790°C). (Online version in color.)
Figure 6 shows the TDZ of Steels D1 and D2 heated at various temperatures for 15 minutes. As seen in the figure, the addition of Nb decreased the decarburization rate. However, the temperature dependence of the decarburization rate was not affected by the Nb addition.

Influence of decarburized temperature on the thickness of decarburized zone of Steels D1 and D2. (Decarburized time: 15 min.) (Online version in color.)
Figure 7 shows the tensile strength of the samples of 1 mm thick heated at 760°C for 5 and 15 minutes at which the decarburization rate is nearly maximum and that of the sample heated at 800°C for 10 s. The latter sample was machined from 1.4 mm to 1 mm in thickness after the heat treatment to remove the possible decarburized layer. As recognized in the figure, the tensile strength decreased by decarburization. The quantitative evaluation of the reduction of the strength will be discussed later.

Influence of decarburization on tensile strength of Steel D1. (Online version in color.)
Figure 8 shows the results of bending tests using the same samples as used in the tensile tests. Bendability remarkably increased by decarburization. However, the improvement of bendability was only slight by extending the decarburization time from 5 min to 15 min. It means that the usual heating time of 5 min in the hot stamping process is a sufficient decarburization time to improve bendability.

Effect of decarburization on critical bending angle of Steel D1. (Online version in color.)
Figure 9 shows the total energy consumed for the bending tests (the integral value of the multiplication of bending load and stroke increment). This data suggests the amount of absorbed energy at the time of the collision and indicates the improvement of the crashworthiness.

Effect of decarburization on total bending energy per unit area of Steel D1. (Online version in color.)
Bendability of ultrahigh strength cold rolled steel sheets can be expected to improve by decarburization. However, since decarburization can be performed only in the heating zone of the continuous annealing line, the available decarburization time is much shorter than that in the hot stamping process. Therefore, the effect of the thickness of the decarburized zone on bendability in the thinner decarburized layer was examined. The result is shown in Fig. 10. The plots marked ● were obtained at a decarburization temperature of 790°C for various holding time and those marked ▲ are obtained at 760°C for 5 min and 15 min. It is recognized that a 7 μm thick decarburized layer improved the bending angle from 55 degrees to 78 degrees by around 40% which is beneficial to bending deformation and crashworthiness of cold rolled high strength steels. However, this possibility has never been explored and applied to cold rolled high strength steels.

Effect of thickness of decarburized zone on critical bending angle of Steel D1. (Online version in color.)
Delayed fracture tests were performed using Steels D1 and D2. Delayed fracture tests are usually carried out with the sample which is machined to a profile given in Fig. 2 after heat treatment. To study the effect of decarburization on the resistance to delayed fracture, a decarburized layer must be formed at the notch of the sample, which is the starting point of fracture. Therefore, the sample was first machined to the given profile and then decarburized. Because the shape of the sample was not rectangular, the direct current heater could not be used for heating. Therefore, the sample was heated in an electric furnace in which the dew point was controlled. The samples to be decarburized were heated at 790°C for 20 min in a 100% hydrogen atmosphere with a dew point of 57°C whereas the reference samples were heated at 790°C for 20 min in a 100% nitrogen atmosphere with a dew point of −38°C. Both samples showed a metallic surface and the noticeable scale was not observed. The samples were lightly pickled with hydrochloric acid and then BH-treated.
The microstructure of the reference and decarburized samples are shown in Fig. 11. The reference samples reveal no decarburized layer, whereas the decarburized samples reveal the thickness of the decarburized zone of 55 μm and 40 μm for Steels D1 and D2, respectively. The suppression of decarburization by the addition of Nb shown in Fig. 6 was confirmed by this experiment as well. The fact that the decarburization layers in this experiment are thicker than those shown in Figs. 5 and 7 indicates that decarburization by dew point control in a non-oxidizing atmosphere occurs more efficiently than that conducted in air.

Microstructure in the vicinity of surface of reference and decarburized samples of Steels D1 and D2 heated at 790°C for 20 min and quenched.
Figure 12 shows the results of the delayed fracture tests. The decarburized sample of D2 did not fracture even after 380 h, so the experiment was stopped there. As can be seen in these results, it has been clarified that decarburization increases the resistance to delayed fracture. In addition, the susceptibility to delayed fracture is reduced by the addition of Nb.

Effect of decarburization on time to delayed fracture of reference and decarburized samples of Steels D1 and D2 heated at 790°C for 20 min and quenched and undergone BH treatment. (Online version in color.)
To understand the decarburization behavior described in Session 3.1, the effects of decarburization temperature and time are discussed using a metallurgical model. Various decarburization models have been developed.19,20,21,22,23,24) Among them we used the model of Choi et al.19) As seen in Fig. 13, the thickness of the decarburized zone is determined by the C flux Jα flowing out into the decarburization zone from the decarburizing interface and the C flux Jγ flowing in from the austenitic matrix into it. However, Jγ becomes zero at the two-phase region temperature. This condition holds at 740°C in Steel D1 and D2. The thickness increment ΔXd per Δt is described by Eq. (1).
| (1) |

Schematic depiction of C distribution in the vicinity of decarburizing interface and the corresponding equilibrium phase diagram. (Online version in color.)
The calculation results together with the experimental results are shown in Fig. 14. Here, Cα, Cγ were determined using Thermo-calc, and for the diffusion coefficients Dα, and Dγ, the following equations given in the published papers25,26) were used.
| (2) |
| (3) |

Evolution of thickness of decarburized zone calculated at various temperatures together with the experimental results. (Online version in color.)
The calculated results of the thickness of the decarburized layer are higher than the experimental values in the high temperature region, but the agreement is good in the low temperature region, especially the experimental result showing no significant difference in the decarburized thickness at 740°C and 760°C was well described.
Analysis of the calculation results shows that although the diffusion rate is high in the high temperature region, Cα representing the concentration gradient in the decarburized region, is low and the (C0−Cγ) representing the concentration gradient in the austenite region is large, so decarburization is disadvantageous in terms of the concentration gradient, resulting in the relatively thin decarburized layer. On the other hand, in the low temperature region, the C concentration gradient in the decarburized zone becomes large, and that in the austenite region small, which is advantageous for decarburization. However, the diffusion rate becomes lower with decreasing temperature, which is disadvantageous for decarburization. This analysis indicates that there exists a maximum decarburizing rate at a certain temperature. The steel studied showed the maximum value at 740°C to 760°C.
The reason why the calculated value was smaller than the experimental value in the high temperature region is discussed. A possible reason may be that the flux of C from the austenite matrix was small in the calculation. Therefore, recalculation was performed using a modified value of Dγ, namely a value obtained by multiplying the used diffusion coefficient by 5. As a result, there was a good agreement between the experimental and calculated values as shown in Fig. 15. Since the concentration gradient in the γ region was small in the low temperature region, there was little effect of Dγ on the flux of C and consequently on the thickness of decarburized layer. The diffusion coefficient initially used was the lattice diffusion coefficient of C in γ. However, the austenite grain size of the sample used is 10 μm or less, and it is presumed that C flows into the interface partially by grain boundary diffusion. The fact that the calculation results using a high diffusion coefficient approached the measured values suggests that grain boundary diffusion may have affected. The result shown in Fig. 16 supports this presumption. The samples of Steel D1 whose austenite grain size was varied by heating to various temperatures from 800°C to 1200°C at a heating rate of 10°C/s were decarburized at 790°C for 20 min. The finer the grain size was, the thinner the decarburized layer was.

Evolution of thickness of decarburized zone calculated with a modified Dγ at various temperatures together with the experimental results. (Online version in color.)

Influence of prior austenite grain size on thickness of decarburized zone. The samples were decarburized at 790°C for 20 min. (Online version in color.)
On the other hand, the decarburization rate was decreased by the addition of Nb. At the decarburization temperatures, Nb exists both in solution and as NbC. Decarburization phenomena have been used for the study on the γ→α transformation kinetics, and the effect of solute dragging and pinning effect on the transformation kinetics has been discussed in detail.27) Both the solute drag force and the pinning force decrease the decarburizing rate. A quantitative evaluation of the effects of the current Nb addition on the decarburizing rate will be reported elsewhere.
4.2. Influence of Decarburization on Tensile StrengthAs shown in Fig. 7, it was found that the strength was significantly reduced by decarburization. This decrease in the strength is discussed in detail with an example of a sample decarburized at 760°C for 15 min. The tensile strength and the thickness of the decarburized zone of the sample are 2026 MPa and 40 μm, respectively. Since the hardness of the decarburized layer is about Hv120, it can be estimated that the tensile strength is about 400 MPa. For the strength of the martensite single-phase material is 2234 MPa as shown in Fig. 7, assuming that the compound rule of Eq. (4) holds, the strength reduction of 121 MPa is estimated due to the formation of the decarburized layer, and the strength of the decarburized sample is calculated to be 2113 MPa. Here, σm is the tensile strength of martensite, σf is the tensile strength of ferrite, A is the total cross-sectional area Am is the cross-sectional area of martensite, and Af is the cross-sectional area of ferrite.
| (4) |
However, this value is considerably higher than the actual strength of the decarburized sample. In order to examine this difference, the hardness near the surface was measured. As shown in Fig. 17, it can be seen that the hardness of the martensite matrix in the vicinity of the decarburizing interface is significantly reduced. This is because, as shown in Fig. 13, C flows from the austenite phase to the decarburization interface as the decarburization proceeds, and a C-decreased zone is formed. The strength reduction of the sample calculated from the hardness reduction of martensite near the decarburization interface is 86 MPa, and if this value is added to the strength reduction due to the decarburization layer, the strength of the decarburized sample is 2027 MPa, which almost corresponds to the actual value. In addition, a marked change in the hardness of martensite near the decarburizing interface indicates that C flows from austenite into the interface with a considerable concentration gradient.

Hardness distribution in the vicinity of the decarburized interface. (Steel D1, The sample was decarburized at 760°C for 15 min.) (Online version in color.)
The reason why decarburization greatly improved bendability is discussed here. Figure 18 shows the state of a crack observed in a bending test sample. The sample was produced in a way that the bending test was stopped when the load dropped 30 N from the maximum load, meaning the initial stage of crack development. The decarburized layer of this sample had a thickness of about 50 μm. The left figure is an overall view of the crack, and the right figure is an enlarged view of the crack initiation position. It is clearly seen that the crack was initiated not from the surface but from the decarburized interface. Moreover, it is recognized that the crack propagated rapidly in the martensite phase, and slowly in the decarburized layer. This observation strongly indicates that the improvement in bendability due to decarburization is due to the formation of a ductile decarburized layer which suppresses the initiation of cracks on the surface and delays cracks to propagate to the surface. In addition, as shown in Fig. 17, the decrease in the hardness of martensite in the vicinity of the decarburized interface is presumed to reduce the susceptibility to cracking additionally.

State of a crack observed in a bending test sample. (The left figure is an overall view of the crack, and the right figure is an enlarged view of the crack initiation position.)
As shown in Fig. 12, the resistance to delayed fracture was significantly improved by the decarburization treatment. As one of the reasons for this improvement in the resistance to delayed fracture, it would be possible that the presence of the decarburized layer suppressed the penetration of hydrogen. To examine this presumption, the amount of hydrogen in the decarburized sample and non-decarburized sample of D1 which were BH-treated and then immersed in a 10% ammonium thiocyanate solution for 1 h and 3 h was measured. As shown in Fig. 19, the presence of the decarburized layer facilitates the penetration of hydrogen into the sample, which cannot explain the improvement in the resistance to delayed fracture due to decarburization. The mechanism for improving bendability explained in the previous section seems to be applicable to explain the improvement in the resistance to delayed fracture. That is, it is assumed that the delayed fracture did not initiate at the surface but at the interface between the decarburized layer and the matrix, and the propagation of cracks was also suppressed due to the presence of the softer surface zone.

Amount of hydrogen stored in the decarburized samples and non-decarburized samples of D1 which were BH-treated and then immersed in a 10%-NH4CN solution for 1 h and 3 h. (Online version in color.)
The resistance to delayed fracture was increased by the addition of Nb. This result is consistent with the results reported earlier.8,9,10,11,12,13,14,15) As the explanation of the increase in the resistance to delayed fracture by the addition of Nb, the following mechanisms are conceivable; ① the grain refinement of martensite, ② an increase in sites at which hydrogen is trapped by precipitated NbC, ③ an increase in the grain boundary coherence by segregated Nb, ④ a decrease in the mobility of dislocations generated from the tip of the crack due to the interaction between the dislocations and Nb28,29,30) and ⑤ the retardation of the clustering of supersaturated vacancies due to the interaction between the vacancies and Nb in solution.28,31)
For the discussion on the mechanisms improving the susceptibility to delayed fracture due to the addition of Nb, a supplemental experiment was performed using the steels whose chemical composition was given in Table 2. To determine the effect of grain refinement, Steel AN without Nb was heated to various temperatures to change the grain size. Figure 20 shows a relationship between the time to delayed fracture and the martensite grain size of steels with and without Nb. The samples were heated at 10°C/s to 825°C and immediately quenched with water, and subsequently BH-treated. The conditions of the conducted delayed fracture tests were the same described in Section 2.4. The deviation of the time to delayed fracture of the Nb containing steels from the dotted line shown in the figure indicates that the improvement in delayed fracture time of the Nb steels cannot be explained by the effect of grain refinement alone.
| C | Mn | Si | P | S | Ti | Nb | Al | B | N | |
|---|---|---|---|---|---|---|---|---|---|---|
| AN | 0.32 | 2.5 | 0.1 | 0.001 | 0.0023 | 0.01 | − | 0.04 | 0.002 | 0.0005 |
| BN | 0.32 | 2.5 | 0.1 | 0.001 | 0.0024 | 0.01 | 0.025 | 0.04 | 0.002 | 0.0005 |
| CN | 0.32 | 2.5 | 0.1 | 0.001 | 0.0023 | 0.01 | 0.05 | 0.04 | 0.002 | 0.0006 |
| DN | 0.32 | 2.5 | 0.1 | 0.001 | 0.0024 | 0.01 | 0.075 | 0.04 | 0.002 | 0.0007 |

Influence of martensitic grain size on time to delayed fracture of the steels with and without Nb addition. (Online version in color.)
Bian et al.8) have reported that finely precipitated NbC trapped hydrogen and consequently contributed to increasing the resistance to delayed fracture of quenched Nb bearing ultrahigh strength steels. This result holds for quenched martensitic steels without BH-treatment. In the present experiments, the samples were, however, BH-treated before delayed fracture tests. An observation of the samples revealed that most of NbC were covered with fine ferrous carbide,14,15) and the amount of stored hydrogen of samples immersed in 10% ammonium thiocyanate solution for 48 h was hardly affected by the Nb content as seen in Fig. 21, meaning that the mechanism ② seems to play a minor role regarding BH-treated steel sheets.

Influence of the Nb content on the total amount of stored hydrogen.
Concerning mechanism ③, Nb enhances the cohesion of grain boundary according to the theoretical calculation of Geng et al.,32) and suppresses the initiation of micro-cracks in the grain boundary and increases the resistance to delayed fracture. However, the quantitative effect is unknown.
Nb is known to have a strong interaction with dislocations and vacancies. Therefore, the mechanisms ④ and ⑤ are expected to play a certain role. However, their quantitative effects on the resistance to delayed fracture have not been clarified.
These considerations indicate that Nb in solution both in the matrix and at the grain boundary contributes to the improvement in the resistance to delayed fracture. The quantitative evaluation of the effects of the various mechanisms is future work.
In this paper, the decarburization behavior, and the effects of decarburization on strength, bendability and delayed fracture susceptibility of ultrahigh strength steel sheets have been investigated. The following results were obtained;
(1) The investigated martensitic steel sheet of 0.35% carbon revealed the maximum decarburization rate at 740°C–760°C.
(2) The addition of Nb reduced the decarburization rate.
(3) A decrease in the strength of the steel sheet due to decarburization was caused not only by the formation of the decarburized layer but also by a decrease in the strength of the martensite matrix near the decarburized interface attributed to the C diffusion in austenite.
(4) The formation of the decarburized layer remarkably improved the bendability of 2000 MPa class steel sheets.
(5) If steel sheets are decarburized under proper conditions during the conventional furnace heating, hot stamped steel parts with excellent bendability can be produced. Further, even in the continuous annealing line where the available decarburization time is usually short, cold rolled ultra-high strength steel sheet with good bendability may be produced because a comparatively thin decarburized layer improves bendability.
(6) The formation of the decarburized layer also remarkably improved the resistance to delayed fracture of 2000 MPa class steel sheets. The mechanism for improving the resistance to delayed fracture is considered to be similar to that for improving bendability.
(7) The Nb addition improved the susceptibility to delayed fracture although the thickness of the decarburized ferrite zone was reduced. Positive effects of the Nb addition on the susceptibility to delayed fracture appear to be due to grain refinement, and Nb in solution both in the matrix and at the grain boundary.
The authors would appreciate the financial support of CBMM in performing this research and also sincerely thank Mr. Masaaki Fujioka, Nippon Steel, for providing the thermodynamic calculation data.