2021 Volume 61 Issue 4 Pages 1315-1321
2021 Volume 61 Issue 4 Pages 1315-1321
In order to suppress global warming, it is necessary to reduce the weight of autobodies, whereas improvement of the collision safety of automobiles is required which results in a weight increase. Research and development of high-strength steel sheets for autobodies are being carried out to achieve both two objectives. Among various high-strength steel sheets, low alloy steels utilizing transformation-induced plasticity of retained austenite (TRIP-aided steels) have attracted attention as body frame and automotive parts materials because of their high strength and ductility. However, there is concern about hydrogen embrittlement in low alloy TRIP-aided steels with a tensile strength exceeding 1000 MPa, as in conventional high-strength steels. In this study, in order to reveal the role of retained austenite for hydrogen embrittlement on TRIP-aided bainitic ferrite steel (TBF steel), (i) several TBF steel sheets with different volume fraction and carbon concentration of retained austenite were prepared by austempering the original sheet for different durations, and (ii) slow strain rate technique tensile tests were carried out on hydrogen-charged and uncharged test pieces of the sheets together with microstructure observations and X-ray diffractions. It was revealed that hydrogen embrittlement of TBF steel sheet became suppressed with increasing austempering time, which was attributable to the increase of surface area of lath and/or filmy metastable retained austenite acting as a trapping site for hydrogen.
Recently, in the automobile industry, the weight of body-in-white has been reduced to improve fuel efficiency, as a measure against global warming. On the other hand, the improvement in collision safety of automobile that results in a weight increase has been required. To fulfil the two conflicting requirements, various high-strength steel sheets have been actively researched and developed in order to achieve both high strength and ductility of body structure materials.1) The tensile strength (TS) level of high-strength steel sheets had been expanded from HSLA steel sheets (TS 600 MPa class), to Dual Phase steel sheets (TS 780 MPa class) and C-Si-Mn-based polygonal ferrite type low alloy transformation-induced plasticity (TRIP2)) steel sheets (TRIP-aided Polygonal Ferrite steel; TPF steel, TS 780 MPa grade), which are the first-generation advanced high-strength steel sheet (AHSS). The weight reduction of the autobody has been achieved by using these first-generation AHSS as the vehicle body material. Around the year of 2000, Quench & Partitioning (Q&P) steel sheet3,4,5) has been developed by Speer et al., and TRIP-aided bainitic ferrite steel (TBF steel), TRIP-aided bainitic ferrite/martensitic steel (TBM steel), and TRIP-aided martensitic steel (TM steel) have been developed by Sugimoto et al.6,7,8,9,10,11) as third-generation AHSSs. These third-generation AHSSs are expected to advance further the weight reduction and improvement of collision safety of autobodies.
Since the TBF steel sheet has excellent press formability, impact toughness, and fatigue properties by TRIP effect, it is further expected as a next-generation AHSS for automobiles. However, as with conventional high-strength steel sheets, hydrogen embrittlement is also a concern in low-alloy TRIP-aided steel sheets with a tensile strength exceeding 1.0 GPa. Several studies on hydrogen embrittlement of TRIP-aided steel sheets have been reported.12,13,14,15) In the previous research,12,13,14) it was reported that the TBF steel has higher hydrogen embrittlement resistance than martensitic steel due to the hydrogen trapping effect of γR. On the other hand, there are many unclear points about the detailed relationship between hydrogen embrittlement and the shape, volume fraction, and stability of γR. It is considered that clarifying the role of γR contributing to the improvement of hydrogen embrittlement resistance of TRIP-aided steel sheets can be utilized for the material design of TRIP-aided steel sheets.
In this study, TBF steel sheets with various γR volume fraction, carbon concentration, shape were prepared by austempering the original sheet for different durations, and the hydrogen embrittlement resistances of the TBF steel sheets were investigated by means of slow strain rate technique (SSRT) tensile test.
A cold-rolled sheet of 1.2 mm in thickness and with the chemical composition presented in Table 1 was used in this study. Before receiving this sheet, hot rolling from 30 mm to 3.2 mm followed by cold rolling to 1.2 mm thickness was conducted. Martensite-transformation-start temperature (Ms) of the sample was calculated by following Eq. (1),16) with the resultant Ms of 376°C,
| (1) |
| C | Si | Mn | Al | Nb | Mo | P | S | N | O | MS |
|---|---|---|---|---|---|---|---|---|---|---|
| 0.40 | 0.49 | 1.51 | 1.02 | 0.05 | 0.20 | <0.005 | <0.005 | 0.0019 | 0.0015 | 376 |
In order to perform a tensile test using the SSRT tensile test machine, the sample steel sheet before heat treatment was machined to a thickness of 0.8 mm by grinding. Then, the tensile test pieces were cut from the sheet by electrical discharge machining. Figure 1 shows the shape and size of the test piece. The test pieces were heat-treated under the five conditions shown in Fig. 2. In order to investigate the effect of the austempering time on the γR volume fraction and stability in the TBF steel sheet, the austempering temperature was set to 425°C and the austempering time was varied from 500 s to 10000 s. The reason for setting the austempering temperature at 425°C was that a high γR volume fraction and carbon concentration were obtained in the TBF steel sheet, which was produced by austempering for 500 s at various austempering temperatures (350–500°C).

Morphology and dimensions of SSRT tensile test piece (in mm).

Heat treatment diagrams of TBF steel sheets. Full austenization treatment at 950°C and austempering at 425°C were made in salt baths.
The SSRT tensile test was performed using a machine (Toshin Kogyo Co.) at a room temperature of 25°C and a strain rate of 1.39 × 10−6 s−1. The tests were conducted under three testing conditions where cathodic hydrogen charging condition and testing environment were changed. Conditions No. 1, No. 2 and No. 3 were test without any hydrogen charging, test in ambient atmosphere with hydrogen pre-charging for 24 h, and test in a hydrogen charging solution with 24 h pre-charging, respectively. The charging was made in a H2SO4 solution of pH 2.5 containing 0.1 mass% NH4SCN. The test piece was immersed in the solution at 25°C, the counter electrode was platinum and the current density was 20 A/m2. The SSRT tensile test was begun within 20 min after hydrogen charging. In the hydrogen charging condition of this study, the amount of diffusible hydrogen in the TBF steel sheet subjected to austempering for 3000 s was 1.85 massppm according to TDS (Thermal Desorption Spectrometry) at a heating rate of 0.0277 K/s using an ESCO EMD-WA1000S/W machine. The amount of charged hydrogen in each test pieces were considered to be almost the same, since the charging conditions have been unified. The hydrogen embrittlement sensitivity (HES) was based on the total elongations of results from SSRT tensile tests. The HES was calculated by the Eq. (2),
| (2) |
The volume fraction of the retained austenite (fγ) was obtained by the integrated intensities of diffraction peaks of (200)α, (211)α, (200)γ, (220)γ, and (311)γ measured by Cu-Kα radiation.17) The carbon concentration in the retained austenite (Cγ) was estimated by Eq. (3)18) using a lattice parameter (aγ (×10−10 m)) measured from the (200)γ, (220)γ, and (311)γ diffraction peaks of Cu-Kα radiation.
| (3) |
| (4) |
Figures 3(a)–3(e) show the results of scanning electron microscopic observation of the TBF steel sheets austempered for durations ranging from 500 to 10000 s. At each austempering time, the matrix of bainitic ferrite is observed. The γR appears to exist in the form of film or needlelike between the matrix phase. In the sheet austempered for 500 s, a blocky structure is observed. Although this blocky structure was described as γR in the research report by Hojo et al.,20) it is possible that a part of the blocky γR was transformed into blocky martensite-austenite constituent structure by oil quenching after short austempering in this study. For austempering time of 1000 s, a small amount of blocky structure is also observed although it is unclear. For the austempering time of 3000 s or more, no blocky structure was confirmed. For the austempering time of 10000 s, γR was decomposed, and a microstructure considered to be perlite is observed.

Scanning electron micrographs of the TBF steel sheets austempered at 425°C for (a) 500 s, (b) 1000 s, (c) 3000 s, (d) 5000 s, and (e) 10000 s. MA, BF, P, and γR with white arrows indicate martensite-austenite constituent structure, bainitic ferrite, perlite, and retained austenite, respectively.
Figures 4(a)–4(d) show the stress-strain curves resulted from SSRT tensile test. At any austempering time, the 0.2% proof stresses of the TBF steel sheets are about 900 MPa and the tensile strength exceed 1000 MPa in test condition No. 1 without hydrogen charging. In this test condition, the total elongations of TBF steel sheets subjected to austempering for 500–5000 s were 28.8% (500 s), 27.4% (1000 s), 31.9% (3000 s), and 27.0% (5000 s), respectively. The total elongation was about 30% for austempering time for 500–5000 s, and the maximum elongation was achieved by austempering for 3000 s. The total elongation of the steel sheet austempered for 10000 s was 24.2%, lower than for the other austempering durations.

Nominal stress-strain curves of the SSRT tensile test in the TBF steel sheets austempered at 425°C for (a) 500 s, (b) 1000 s, (c) 3000 s, and (d) 5000 s.
In the test condition No. 2, the total elongations of the TBF steel sheets subjected to austempering for 500–5000 s were 1.9% (500 s), 19.6% (1000 s), 26.4% (3000 s), and 25.1% (5000 s), respectively. As the austempering time increased, the decrease in ductility of the TBF steel sheet due to hydrogen was suppressed.
The total elongations of the TBF steel sheets 24 h pre-charged and subsequently tested in a hydrogen charging solution were 1.7% (500 s), 4.6% (1000 s), 2.7% (3000 s), and 3.8% (5000 s), respectively. Compared to the results in test condition No. 2, total elongations in test condition No. 3 were significantly decreased.
Figures 5(a)–5(e) show images of fracture surface after testing in condition No. 1, and Figs. 5(f)–5(j) show images of fracture surface after testing in condition No. 2. In Figs. 5(a)–5(e), fracture surfaces with dimples were observed on the entire fracture surface, an example of which is shown in the close-up image in Fig. 5(c). In Figs. 5(f)–5(j), quasi-cleavage fracture surfaces were observed around the corners of the cross-sectional area of the test pieces as shown in the close-up image in Fig. 5(h). The ratio of the quasi-cleavage fracture surface to the entire fracture surface decreased with increasing austempering time. These observations were consistent with the results of the total elongation in the SSRT tensile test, and it is found that the decrease in ductility due to hydrogen can be suppressed as the austempering time increases. The fracture surfaces of the test pieces in test condition No. 3 could not be observed because the fracture surfaces were corroded by the hydrogen charging solution after fracture.

Scanning electron micrographs of fracture surface of the TBF steel sheets austempered at 425°C for (a, f) 500 s, (b, c, g, h) 1000 s, (d, i) 3000 s, and (e, j) 5000 s. (a–e): test condition No. 1 (without pre-charging). (f–j): test condition No. 2 (with 24 h pre-charging). (c, h): high magnification images of white square area in Figs. 5(b) and 5(g), respectively. Dashed regions show quasi-cleavage fracture surface.
Figures 6(a) and 6(b) show scanning electron micrographs of cross-sectional area near the fracture tip of the test piece after SSRT tensile test in condition No. 2. In the test piece austempered for 500 s, cracks are observed at the periphery of or inside the blocky structures. On the other hand, cracks of the test piece austempered for 3000 s are observed at the interface between bainitic ferrite and γR. According to these results, it seems that a fracture origin of hydrogen embrittlement in the TBF steel sheets austempered for 500 and 1000 s was the MA structure. In the TBF steel sheets austempered for 3000 and 5000 s, the fracture of hydrogen embrittlement might have originated at the interface between bainitic ferrite and γR.

Scanning electron micrographs of cross-sectional area near fracture tip of test piece austempered for (a) 500 s and (b) 3000 s after SSRT tensile test in condition No. 2 (with 24 h pre-charging). Arrows in the figure indicate cracks. Tensile direction is parallel to L direction.
Figure 7 shows the initial volume fraction and carbon concentration of γR of the specimens. Both the volume fraction and carbon concentration include those of γR of any morphologies (filmy γR, blocky γR and particulate γR in MA structure). Although the volume fraction of γR decreases at 1000 s, it is increased by longer austempering (3000 s and 5000 s), but the XRD peaks of γ was not observed clearly in the specimen austempered for 10000 s. This is consistent with the microstructure observation result that γR was decomposed into ferrite and carbides in this specimen, which means that γR cannot be retained for such a prolonged austempering. The carbon concentration of γR tends to decrease slightly from austempering time of 500 s to 5000 s, but it is considered that the carbon enrichment of γR in these specimens is enough for exerting the TRIP effect.

(a) Initial volume fraction (fγ0) and (b) carbon concentration (Cγ0) of γR of TBF steel sheets austempered at 425°C for 500–10000 s.
Figures 8(a) and 8(b) show the stability of γR against tensile deformation without hydrogen charging and with hydrogen pre-charging for 24 h, respectively. Table 2 shows plastic strains εp used in Eq. (4) for calculations of k value in Fig. 8. The specimen without hydrogen charging has higher stability of γR (smaller k value) than that with pre-charging, at any austempering time, but the differences are generally small except for austempering time of 500 s. The lowest stability of γR of the specimen austempered for 500 s in test condition No. 2 can be attributable to the significantly small total elongation in the SSRT tensile test, resulting in of the extremely small plastic strain in calculating stability of γR, compared to the other specimens.

Volume fraction of γR (fγ0: initial volume fraction, fγ: volume fraction after the plastic strain εp (shown in Table 2) is applied) and k values in TBF steel sheets. (a) test condition No. 1: without hydrogen charging. (b) test condition No. 2: with 24 h hydrogen pre-charging.
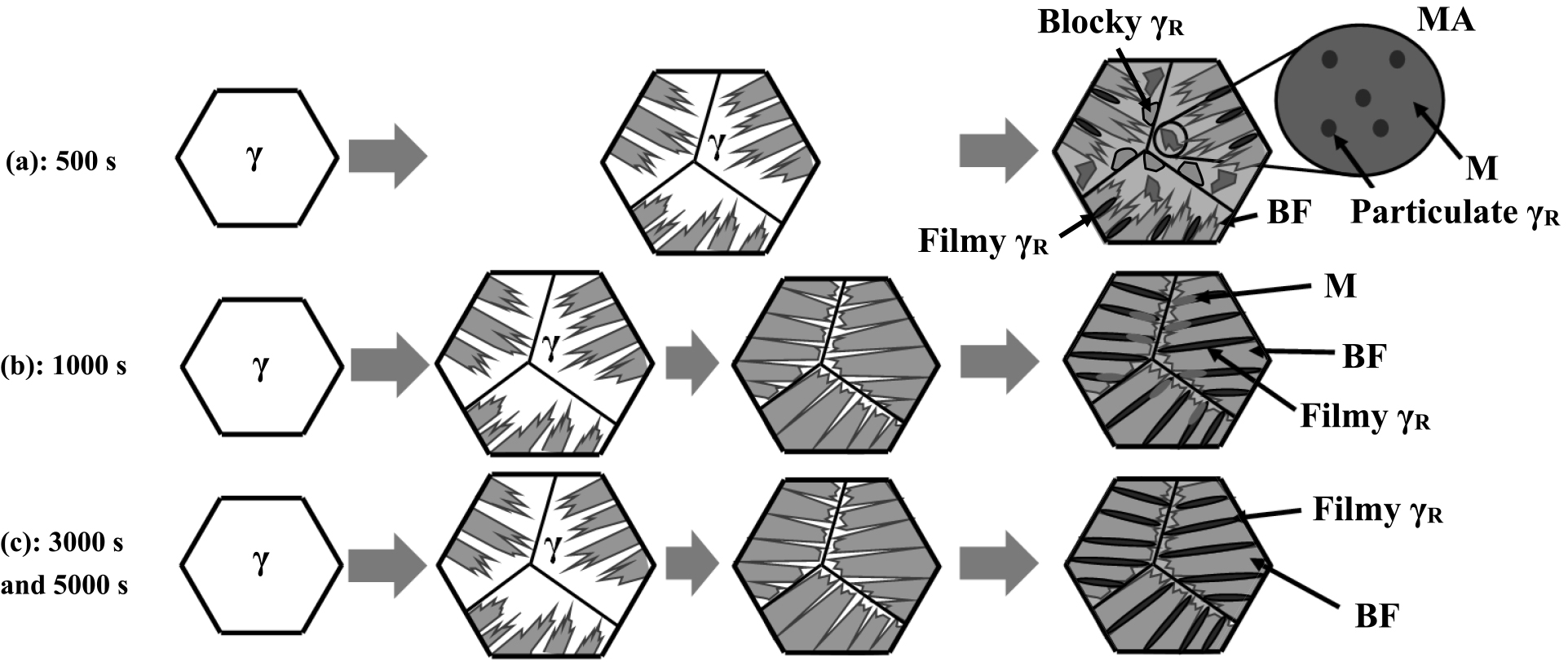
Table 3 summarizes the microstructural characteristics of γR and the matrix, and the hydrogen embrittlement sensitivity (HES) of the specimens in this study. Figure 9 shows the illustrations of microstructural changes during austempering. For austempering time of 500 s, blocky MA (martensite-austenite constituent as a secondary structure) structure were observed in the bainitic ferrite matrix. As mentioned earlier, this blocky MA structure was considered to be a product of partial phase-transformation from untransformed γ by cooling after austempering, and to be a mixed structure of martensite and fine particulate γR, as reported by the previous studies.7,21,22) Although MA structure includes a small amount of γR, it may be mainly mounted in the martensite (Fig. 9(a)).
| Austempering time | fγ0 (vol%) | Cγ0 (mass%) | Morphology of γR | Estimated surface area of filmy γR (μm2) | Location of γR | Matrix and secondary structure | HES (%) |
|---|---|---|---|---|---|---|---|
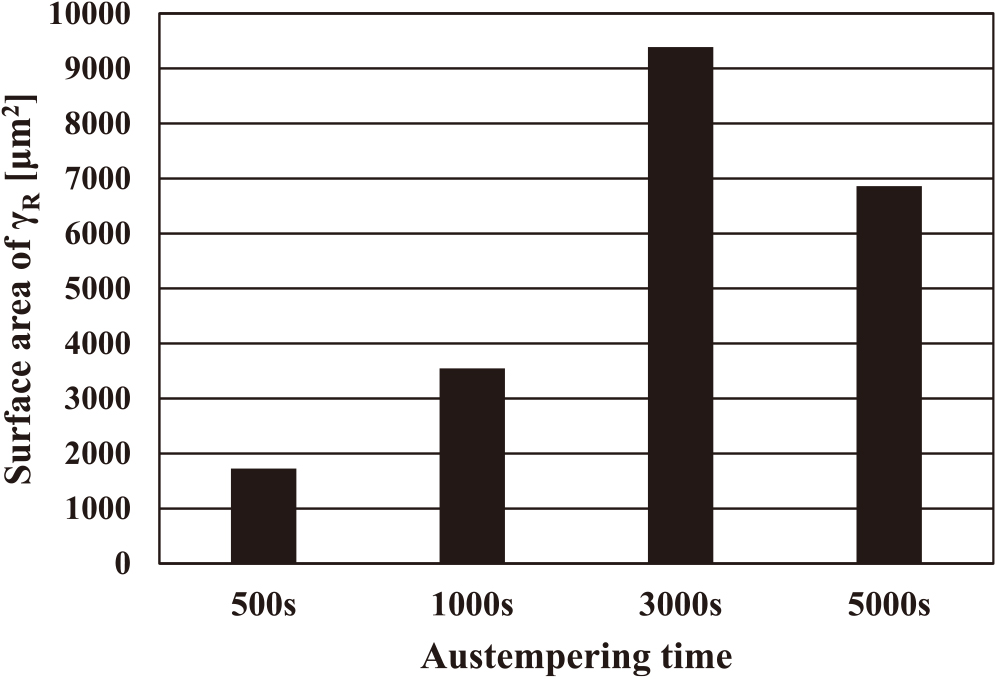
| A: 500 s | 19.2 | 1.6 | Blocky, filmy | 1727 | BF boundary and inside of MA | BF + blocky M | 93.4 |
| B: 1000 s | 13.7 | 1.5 | Filmy | 3549 | BF boundary and inside of MA | BF + small amout blocky M | 28.3 |
| C: 3000 s | 21.6 | 1.5 | Filmy | 9388 | BF boundary | BF | 17.1 |
| D: 5000 s | 21.3 | 1.2 | Filmy | 6860 | BF boundary | BF | 5.3 |
fγ0: Initial volume fraction of γR, Cγ0: Initial carbon concentration of γR, HES: Hydrogen embrittlement sensitivity based on the total elongations in Fig. 4.
Illustrations of microstructural change of specimens during austempering.
On the other hand, the matrix austempered for 1000–5000 s is composed of bainitic ferrite (Figs. 9(b) and 9(c)), though the microstructure austempered for 1000 s includes a small amount of blocky MA structure (Fig. 9(b)). This is probably because the bainite transformation proceeded with enough austempering time, stabilized γ remained as γR without being transformed into martensite.
4.2. Hydrogen Embrittlement ResistanceThe decrease in total elongation of the TBF steel sheets by the hydrogen pre-charging for 24 h was suppressed by increasing the austempering time ranging from 1000 to 5000 s. According to the previous studies,12,13) hydrogen is trapped inside γR and at the boundary of bainitic ferrite lath structure. These hydrogen trapping sites, especially γR, should suppress the diffusion of hydrogen to the prior γ grain boundary and the intergranular fracture. Among the austempering durations of 1000, 3000, and 5000 s in this study, the volume fraction of γR was increased with increasing the austempering time. Therefore, the increase in volume fraction of γR improves the hydrogen embrittlement resistance of the TBF steel sheet, as well as previous study.
However, the hydrogen embrittlement resistance was lower in the test piece austempered for 500 s, despite the highest γR volume fraction. In the present study, blocky γR structure remained at the austempering time of 500 s, which transformed into MA structure during subsequent oil quenching. Hydrogen embrittlement cracking easily occurred at the MA structure (Fig. 6(a)), which is attributable to the higher hardness of the MA structure resulting in higher hydrogen embrittlement sensitivity than the matrix. Although the blocky structures cause a high-volume fraction of γR in the TBF steel sheet, the particulate γR inside the blocky structures does not contribute to the improvement of hydrogen embrittlement resistance because it has a smaller surface area than the filmy γR. The blocky γR that remains in the specimen austempered for 500 s has low stability, and enhances hydrogen embrittlement due to the strain-induced phase transformation from austenite to martensite.22) Therefore, the hydrogen embrittlement resistance was lower in the specimen austempered for 500 s due to MA structure with high sensitivity of hydrogen embrittlement and blocky γR with low stability.
In order to investigate the effect of surface area of the filmy γR that contributes to the improvement of hydrogen embrittlement resistance, the γR surface area in a single grain was roughly estimated (Fig. 10). In Fig. 10, the surface area of the filmy γR along a bainitic ferrite was estimated from the SEM images in Fig. 3 (observed area: approximately 1000 μm2) by assuming the bainitic ferrite to be an ellipsoid. In this estimation, the fine particulate γR involved in the MA structure was excluded, because it was difficult to estimate the surface area of the fine particulate γR involved in the MA structure from SEM images and the MA structure promotes the hydrogen embrittlement as mentioned above. These values of γR surface area shown in Fig. 10 are not absolute values but are sufficient to observe the tendency. The surface area of filmy γR austempered for 500 s was the smallest, and that for 3000 s was the largest among all the austempering conditions. From this result, it is considered that increasing filmy γR surface area, rather than increasing γR volume fraction, is effective for improving the hydrogen embrittlement resistance of the TBF steel sheet. The fine particulate γR inside the MA structure has a smaller surface area than the filmy γR. Therefore, it is presumed that the fine particulate γR does not work sufficiently for hydrogen embrittlement resistance. To improve the hydrogen embrittlement resistance of TBF steel sheet, it is considered that increasing the surface area of filmy γR is required.
Estimated result of the surface area of the filmy γR of TBF steel sheets austempered at 425°C for 500–5000 s.
In the present study, to clarify the role of γR contributing to the improvement of hydrogen embrittlement resistance of TRIP-aided bainitic ferrite (TBF) steel sheets, the hydrogen embrittlement resistances of the TBF steel sheets austempered for different durations were investigated by means of slow strain rate technique (SSRT) tensile test. The results are summarized as follows.
(1) The decrease in ductility of the TBF steel sheet due to hydrogen was suppressed by austempering at 425°C for 1000–5000 s. The hydrogen embrittlement resistance of the specimen austempered for 500 s was lower, despite having high γR volume fraction. These differences of the hydrogen embrittlement resistance of the TBF steel sheet might be caused by differences of microstructural characteristics of γR.
(2) Blocky γR and martensite-austenite constituent were mainly observed in the specimen austempered for 500 s. On the other hand, filmy γR was observed in the test piece austempered for 1000–5000 s. It was considered that increasing the filmy γR surface area as hydrogen trapping site is effective for improving the hydrogen embrittlement resistance of the TBF steel sheet.