2021 Volume 61 Issue 6 Pages 1835-1841
2021 Volume 61 Issue 6 Pages 1835-1841
To alleviate the environmental pressure from the massive discharge of red mud (RM), and develop an economical and environment-friendly ferromanganese desiliconization and dephosphorization process, a novel RM-based desiliconization and dephosphorization flux for ferromanganese was designed and verified through thermodynamic analyses and high-temperature experiments. The results showed that the designed RM-based flux had good melting property, desiliconization capability, and dephosphorization capability after adjusting the experimental temperature and the lime:RM ratio (wt%). Especially, when the temperature was 1623 K and the lime:RM ratio (wt%) was 0.5, the best desiliconization and dephosphorization effects (desiliconization rate of 77.24% and final silicon content of 0.28%, dephosphorization rate of 31.89% and final phosphorus content of 0.31%) were achieved in the situation of high manganese content (≥63.5%). Also, the final slag could continue to remove phosphorus from low silicon ferromanganese. This work verified the feasibility of applying the RM-based flux to the ferromanganese desiliconization and dephosphorization process, which has significant environmental and economic benefits.
Ferromanganese is extensively used as a deoxidizer or alloy additive in steelmaking, but it is one of the main sources of phosphorus contamination in steel.1) The [P] content in blast furnace ferromanganese is usually between 0.4% and 0.6%, which cannot meet the quality requirement for smelting clean steel. To reduce the [P] content from ferromanganese, a lot of researches on the ferromanganese thermodynamic properties and the design of dephosphorization slags for ferromanganese have been conducted in recent years. The thermodynamic research indicates that both ferromanganese and steel require pre-desiliconization before dephosphorization under an oxidizing atmosphere. However, the removal of [Si] and [P] from ferromanganese is more difficult than that from steel due to the strong binding force of [Mn]-[Si] and [Mn]-[P], and the preferential oxidation of [Mn].2,3,4,5,6) The strong oxidization, high alkalinity, and low melting point of slag are key factors for effective desiliconization and dephosphorization. Generally, the CaO–SiO2–FetO slag formed during the steelmaking process cannot effectively remove [Si] and [P] in ferromanganese due to its high melting point and insufficient alkalinity. Hence, CaF2/BaF2 as fluxes and BaO/Na2O7,8,9,10) as high-efficiency fixatives are usually introduced into the slag to improve the desiliconization and dephosphorization effects of ferromanganese. However, the high cost of BaO/Na2O and the subsequent environmental pollution caused by fluoride11,12) were neglected. There is still no economical and environmental desiliconization and dephosphorization slag of ferromanganese to date that can be used in industry.1) Recently, to conserve energy and protect environment, the utilization of solid wastes rich in FetO, such as basic oxygen furnace dust13) and sludge,14) have been widely developed in the steelmaking process, while hardly reported in the ferromanganese smelting process. If a solid waste contains FetO, BaO/Na2O and other components that can be used as fluxes, it is expected to be used in the industry as ferromanganese desiliconization and dephosphorization flux.
Bayer red mud (RM) is an industrial by-product from the caustic (NaOH or Na2CO3) leaching of bauxite to produce alumina. At present, the most conventional method to produce alumina is the Bayer process.15) The production of per ton Al2O3 will generate 0.3–2.5 tons of RM. The annual global production of RM is approximately 150 million tons,16) and the global inventory of 3 billion tons of RM is awaiting for massive utilization.17) Over the years, the RM has been utilized in building,18,19) environmental protection,20,21,22) industrial catalysts,22,23,24) soil amendments,25) etc. Nevertheless, the aforementioned approaches can only consume less than 15% RM. Currently, most of the RM is preserved in landfills or sea, resulting in serious environmental problems. To mitigate the storage problem of RM, it is urgent to develop a new and efficient method to utilize RM. The compositions of RM depend on the chemical and mineralogical constituents of bauxite and the bauxite treatment technologies. The typical compositions of Bayer RM are as follows: Fe2O3(30–60%), Al2O3(10–20%), SiO2(3–50%), Na2O(2–10%), and other minor components.26) The Bayer RM has strong oxidization because of its high Fe2O3 concentration. Na2O in the Bayer RM is a well-known strong desiliconization and dephosphorization flux.27,28,29,30,31) Besides, previous studies have demonstrated that Na2O/Al2O327,28) could be fluxes replacing CaF2/BaF2 to improve the fluidity of CaO–SiO2–FetO slag. Thus, these components in the Bayer RM are very useful in the desiliconization and dephosphorization process. However, the application of RM in the ferromanganese desiliconization and dephosphorization process has never been reported.
In this work, the desiliconization and dephosphorization experiment for ferromanganese was conducted using RM-based flux, and the melting properties of slag and the migration of [Si], [P], [Mn], and [C] in the slag-metal system were studied. The obtained data can give important information for promoting the industrial application of RM-based flux in the ferromanganese desiliconization and dephosphorization process.
Table 1 shows the composition of Bayer RM. The RM has strong oxidization because of its nearly 50% iron oxide content, and the strong oxidization is beneficial for desiliconization and dephosphorization. Besides, the high alkalinity of slag is also a key factor for desiliconization and dephosphorization. To further improve the alkalinity of slag, it is considered to adjust the composition of RM by adding CaO.
| CaO | Al2O3 | Fe2O3 | SiO2 | Na2O | TiO2 | MgO | K2O | LOSS |
|---|---|---|---|---|---|---|---|---|
| 0.85 | 15 | 49.28 | 9.62 | 6.05 | 4.24 | 0.19 | 0.08 | 14.69 |
It is worth noting that the manganese loss of ferromanganese must be as small as possible while removing silicon and phosphorus. The relevant chemical reaction that controls the distribution of silicon and manganese between the slag and ferromanganese is shown in Eq. (1):
| (1) |
| (2) |
| (3) |
| (4) |
The equilibrium constant in Eq. (2) can be written as a function with the distribution ratios and the activity coefficients according to Eqs. (2), (3), (4), as follows:
| (5) |
| (6) |
| (7) |
| (8) |
| (9) |
| (10) |
| (11) |

Effect of CaO: RM ratio (wt%) on

Effect of CaO: RM ratio (wt%) on
Strong oxidization and high alkalinity in the RM-based flux can promote the desiliconization reaction and dephosphorization reaction, and good slag fluidity can accelerate these reactions. Referring to the composition of RM, the isothermal liquidus lines of CaO-SiO2-Fe2O3-13%Al2O3-5%Na2O-3%TiO2 system were calculated by using thermodynamic software Factsage 8.0, as presented in Fig. 3. With the increase of CaO:RM ratio (wt%), the melting point of slag decreases first and then increases. To verify the real melting properties of RM-based flux with different lime (CaO, reagent grade oxide) contents, the hemispherical softening point temperature and flowing temperature of the mixed slag were measured by using Melting Point and Rate Measuring Instrument. The relevant results are shown in Fig. 4. When the lime:RM ratio (wt%) of mixed slag was between 0.2 and 0.5, the hemispherical softening point temperature and flowing temperature were below 1573 K, which meet the temperature requirement (1573–1673 K) in the ferromanganese desiliconization and dephosphorization process. Considering removing [Si], removing [P] and retaining [Mn] capabilities, and the melting properties of the RM-based flux simultaneously, the appropriate CaO:RM ratios (wt%) are 0.4 and 0.5.

Isothermal liquidus lines of the CaO-SiO2-Fe2O3-13%Al2O3-5%Na2O-3%TiO2 slag system. (Online version in color.)

Effect of lime: RM ratio (wt%) on the melting properties of RM-based flux. (Online version in color.)
The ferromanganese desiliconization and dephosphorization experiments were conducted in a silicon molybdenum furnace. First, an alumina crucible (Φ5 cm) loaded with the ferromanganese (200 g) was heated under a purified argon atmosphere to the experiment temperature. After reaching the experiment temperature and keeping for a while, the RM and lime mixture slag (20 g) pressed into blocks was introduced to the ferromanganese, and this time was marked as 0 minutes. Another mixed slag (20 g) was introduced at 30 minutes, and the experiment was terminated at 60 minutes. Ferromanganese samples were collected at 0 minutes, 30 minutes, and 60 minutes with quartz tubes (Φ4 mm), and slag samples were collected at 30 minutes and 60 minutes with iron spoons, respectively. The elements in ferromanganese were determined by silicon molybdenum blue colorimetry (Si), phosphorus molybdenum blue colorimetry (P), carbon and sulfur analyzer (C), and atomic absorption spectrometry (Mn), respectively. The slag composition was analyzed by fluorescence spectrometry. Table 2 shows the specific experimental arrangements. Taking into account that the consumption of FeO content in slag may cause a higher slag melting point, the experimental temperatures were 1623 K and 1673 K.
| Number | T (K) | lime:RM ratio (wt%) | slag:metal ratio (wt%) | ferromanganese initial composition (wt%) | |||
|---|---|---|---|---|---|---|---|
| Si | Mn | P | C | ||||
| No. 1 | 1623 | 0.4 | 10% + 10% | 1.17 | 68.9 | 0.44 | 6.22 |
| No. 2 | 1623 | 0.5 | 10% + 10% | 1.25 | 68.5 | 0.45 | 6.12 |
| No. 3 | 1673 | 0.5 | 10% + 10% | 1.20 | 70.3 | 0.46 | 6.23 |
Figures 5 and 6 show the variations of the element concentrations in ferromanganese and the component concentrations in slag with time under different conditions during the experimental process. When the RM-based flux was introduced in the ferromanganese at 1623 K, [Si] and [Mn] were rapidly oxidized in 0 to 30 minutes, [Si], [Mn], and [P] were partially oxidized in 30 to 60 minutes, and [C] was hardly oxidized. When the temperature was increased from 1623 K to 1673 K, [C] was obviously oxidized between 30 and 60 minutes.
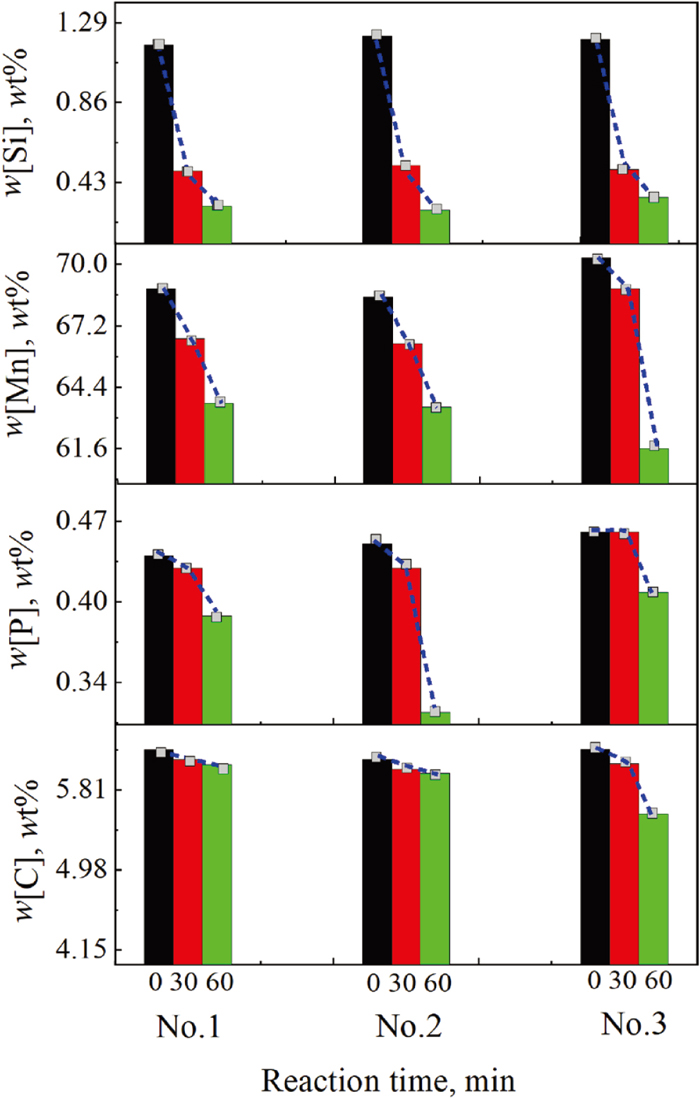
Variations of the element concentrations in ferromanganese with reaction time. (Online version in color.)

Variations of the component concentrations in slag with reaction time. (Online version in color.)
The iron oxide in slag gradually reacted with [Si], [Mn], and [P] in the ferromanganese, thus resulting in the visible variation of slag component concentrations. A large amount of SiO2 and MnO were generated into slag, and FeO in the collected slag was exhausted, as shown in Fig. 6. However, no visible crusting phenomenon was found in the slag during the experiment. Variations of the component concentrations in slag in the process of the experiment were marked in CaO–SiO2–Fe2O3–MnO–(Al2O3–Na2O–TiO2) phase diagram, as shown in Fig. 7. The composition change of RM-based flux was always within the liquid phase region. Therefore, the RM-based fluxes had good slag fluidity during the entire experimental process.
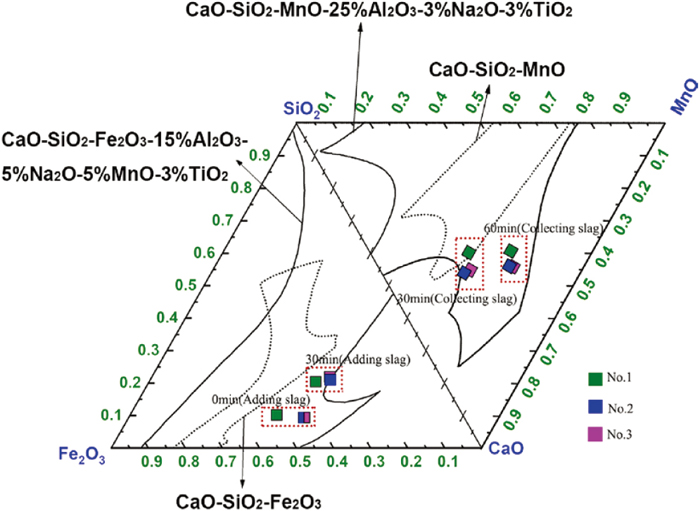
Isothermal liquidus lines of the CaO–SiO2–Fe2O3–MnO–(Al2O3–Na2O–TiO2) slag system at 1623 K. (Online version in color.)
The element concentration in the ferromanganese and the component concentration in the slag can’t truly represent the slag-metal interaction due to continuous changes in the total amount of ferromanganese and slag. Hence, the desiliconization rate (ηSi) was calculated according to Eq. (12), where (mSi)final slag was the weight of [Si] in the final slag; (mSi)initial slag was the weight of [Si] in the initial slag; [mSi]initial ferromanganese was the weight of [Si] in the initial ferromanganese. Figure 8 shows the variations of the ηSi and LSi under different conditions. The RM-based flux had strong desiliconization capability, and the desiliconization rates were all greater than 70%. Compared with the lime:RM ratio (wt%), the temperature caused a more obvious influence on the desiliconization effect. With the temperature increasing from 1623 K to 1673 K, the silicon distribution ratio decreased from 51.8 to 40.8.
| (12) |

Variations of ηSi and LSi under different conditions. (Online version in color.)
Referring to Eq. (12), the calculation of dephosphorization rate (ηP) was similar to that of the desiliconization rate. Figure 9 shows the variations of ηP and LP under different conditions. The dephosphorization rate decreased with the increase of temperature from 1623 K to 1673 K, and the dephosphorization rate increased with the increase of lime:RM ratio (wt%) from 0.4 to 0.5. Especially, when the temperature was 1623 K and the lime:RM ratio (wt%) was 0.5, the best dephosphorization effect (dephosphorization rate of 31.89% and final [P] content of 0.31%) was achieved in the situation of high [Mn] content of 63.5%. To verify the subsequent dephosphorization ability of the RM-based flux, the final slag of No. 2 at 60 minutes was introduced into low silicon ferromanganese at high temperature. The mass ratio of slag and ferromanganese was 10%. Figure 10 shows that the final slag does have further desiliconization and dephosphorization capabilities. The [Si] content decreased to about 0.2%, thereafter, remained constant, and the [P] content decreased in 0 to 45 minutes. The contents of [C] and [Mn] were significantly unchanged. In summary, RM-based flux had good melting properties, desiliconization capability, and dephosphorization capability after adjusting the experiment temperature and the lime:RM ratio (wt%).

Variations of ηP and LP under different conditions. (Online version in color.)

Variations of the element concentrations in low silicon ferromanganese with reaction time.
During the experiment, the [Mn] loss was divided into the oxidation loss and the volatilization loss. The [Mn] loss rate (ηMn loss) was calculated by Eq. (13), where ηMn oxidation was [Mn] oxidation rate; ηMn volatilization was [Mn] volatilization rate; (mMn)final slag was the weight of manganese in final slag; [mMn]initial ferromanganese was the weight of manganese in initial ferromanganese; {mMn}volatilization was the weight of manganese volatilized. Figure 11 shows the variations of ηMn oxidation, ηMn volatilization, and LMn under different conditions. When the lime:RM ratio (wt%) increased from 0.4 to 0.5, ηMn oxidation slightly decreased from 6.12 to 5.93 and LMn decreased from 0.39 to 0.34. Suito et al. also found that LMn decreased with the increase of CaO content in slag.42,43) The temperature had no obvious effects on ηMn oxidation, but it influenced ηMn volatilization significantly. When the temperature increased from 1623 K to 1673 K, ηMn volatilization increased from 2.34 to 5.20. The reasons are as follows: First, the vapor pressure of manganese increased with the increase of temperature; Second, Shao et al. found that a large amount of CO bubbles overflow could take away gaseous Mn.44) Wu45) et al. found the oxidation manganese loss was a negative value when 20–30% manganese oxide existed in slag. The MnO content of 22–25% was generated in the final experimental slags, and the capabilities of removing phosphorus and retaining manganese could be achieved by using the No.2 final slag to refine low silicon ferromanganese. Therefore, methods, such as decreasing the temperature, increasing the lime:RM ratio (wt%), and adding an appropriate amount of MnO, could be considered to further reduce [Mn] loss.
| (13) |

Variations of ηMn oxidation, ηMn volatilization, and LMn under different conditions. (Online version in color.)
In this study, a novel RM-based desiliconization and dephosphorization flux for ferromanganese was designed and verified through thermodynamic analyses and high-temperature experiments. The designed RM-based flux had good slag fluidity, desiliconization capability, and dephosphorization capability after adjusting the experimental temperature and the lime:RM ratio (wt%). The specific results are as follows.
(1) The appropriate CaO:RM ratios (wt%) were determined to 0.4 and 0.5 through the calculation of removing silicon, removing phosphorus, and remaining manganese capabilities, and the study on melting properties of the slag.
(2) When the RM-based flux was introduced in the ferromanganese at 1623 K, [Si] and [Mn] were oxidized first, then [P] was oxidized, while [C] was hardly oxidized.
(3) When the experimental temperature was reduced from 1673 K to 1623 K, the oxidation of [C] and the volatilization of [Mn] were significantly inhibited, and the removal of [Si] and [P] was promoted.
(4) High desiliconization rates (>70%) with low [Si] contents (about 0.3%) were obtained experimentally when the lime:RM ratio (wt%) was between 0.4 and 0.5. Increasing the lime:RM ratio (wt%) from 0.4 to 0.5 could promote removing [P] significantly and inhibit oxidating [Mn] slightly.
(5) Especially, when the temperature was 1623 K and the lime:RM ratio (wt%) was 0.5, the best desiliconization and dephosphorization effects (desiliconization rate of 77.24% and final [Si] content of 0.28%, dephosphorization rate of 31.89% and final [P] content of 0.31%) was achieved in the situation of high Mn content (≥63.5%). Also, the final slag could continue to remove phosphorus from low silicon ferromanganese.
(6) Methods, such as decreasing the temperature, increasing the lime:RM ratio (wt%), and adding an appropriate amount of MnO, could be considered to further reduce [Mn] loss.
Financial support to this project is provided by the National Natural Science Foundation of China (No. 51874082) and NSFC-Liaoning Joint Fund (No. U1908224).