2021 Volume 61 Issue 6 Pages 1768-1774
2021 Volume 61 Issue 6 Pages 1768-1774
This article proposes a structural thermodynamic model of slags to estimate the sulphide capacity (CS) of ternary silicate melts. Sulphide ion (S2−) is incorporated into the silicate structure by substituting quasi-lattice sites in the slag for free oxygen ions (O2−). This structural model can take into account the effect of substituting one metal oxide for another in ternary systems, since it considers that each metallic oxide produces a de-polymerisation reaction of O° + O2− = 2 O− with a characteristic free energy change. The Cs of ternary silicates can be calculated by this model solely from the data of the binary sub-systems − no ternary terms are required. A good agreement was obtained between the experimental and the model results for the ternary slags of the SiO2–CaO–MgO–MnO–FeO system.
The understanding of the thermodynamics of slags has become extremely important due to the higher demands on steel qualities in terms of the presence of impurities such as sulphur.1,2,3) The sulphide capacity (CS) of liquid slag systems has been extensively studied, and many models related to this have been developed. Zhang et al.4) developed an empirical model based on optical basicity to calculate the CS of metallurgical molten slags. Thermodynamic models were also proposed by Reddy and Blander5) and Pelton et al.,6) where CS is calculated solely from the knowledge of thermodynamic activity. Derin et al.7) proposed a neural network approach to estimate the CS in binary and multi-component melts. Choi and Min8) studied the cationic effect of ferrous ions on the CS of CaO–FetO–Al2O3–SiO2 slags from the point of view of the ionic structure within them using micro-Raman spectroscopy. Moosavi-Khoonsari and Jung9) studied the dissolution behaviour of sulphur in silicate melts using the modified quasi-chemical model in the quadruplet approximation.
Reddy and Blander10) proposed that the sulphide ions (S2−) in the dilute solution in silicate melts substitute O2− ions. The equilibration reaction may be written as follows:
| (1) |
The equilibrium constant KM can be derived using the following equation:
| (2) |
Here, aMS and aMO are the activities of MS and MO, respectively, and pO2 and pS2 are the equilibrium partial pressures.
Fincham and Richardson11) defined the CS of a slag system as follows:
| (3) |
Here, (mass pct S) is the mass percentage of dissolved sulphur. The combination of Eqs. (2) and (3) gives the following:
| (4) |
Structure-based models have been developed recently with a consideration of the fact that the structure of silica network breaks down upon the addition of basic oxides, creating a more depolymerised structure. These models assume that silicate melts contain three types of oxygen: (1) bridging oxygen bonded to two silicon atoms (O°), (2) non-bridging oxygen bonded to only one silicon atom (O−), and (3) free oxygen bonded to no silicon atom (O2−).
| (5) |
| (6) |
In a binary solution − SiO2−MO (M = Ca, Fe, Na2, etc.) − the parameters NO2-, NO- and NO° represent the number of moles of various oxygen species per mole of a solution (nMO + nSiO2). Here, it is assumed that every silicon atom is bonded to four oxygen atoms. Thus, mass balance considerations require the following equations:
| (7) |
| (8) |
Here, XSiO2 and XMO are the mole fractions of the oxides.
The present model uses the structural model that was proposed by Lin and Pelton12) and was, subsequently, extended by Romero and Pelton13) to estimate the concentration of the types of oxygen in binary and ternary silicate systems. The structural model has been used to calculate the thermodynamic properties and the phase diagrams for binary and ternary systems.14) This model has also been extended to predict other properties, such as viscosity15,16) of binary and ternary systems and the sulphide capacity (CS) of binary silicate systems.13) The only adjustable parameters introduced to represent the measured CS values within the experimental error limits were small activity coefficients for the sulphides. This model can calculate the activity of the sulphide components, and thus, the CS is, in a way, consistent with all the other properties of the system.
The following model has been developed for the ternary silicate system (SiO2-AO-BO) plus sulphur. The depolymerisation reaction (5) is associated with Gibbs energy change containing enthalpic (ω) and entropic (η) terms for each metallic oxide:
| (9) |
| (10) |
Here, ω and η are expanded as polynomials in terms of XSiO2:
| (11) |
| (12) |
The coefficients ωi and ηi are the parameters of the thermodynamic model, which are obtained by the data optimization.
The present model considers that sulphide ions (S2−) can substitute free oxygen ions (O2−), indicating that the ternary silicate system plus sulphur can be represented as a square pyramid with SiO2 at the apex level, and, as shown in Fig. 1, the base represents a reciprocal ternary subsystem A, B//O, S.
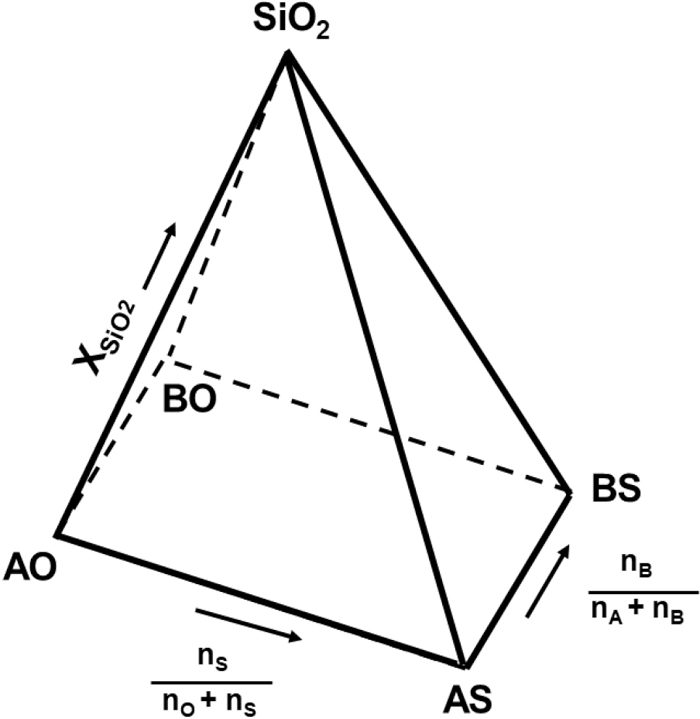
Composition pyramid of the ternary silicate system plus sulphur.
The thermodynamic model considers the following terms:
2.1. Configurational Entropy TermConfigurational entropy (Sc) is physically related to the number of ways particles themselves can be distributed in space. The configurational entropy of the melt is calculated through the multiplicity of the random distribution of
| (13) |
Here, R is the gas constant, and N° is Avogadro’s number.
| (14) |
Here,
| (15) |
| (16) |
| (17) |
| (18) |
| (19) |
The depolymerisation reactions (9) and (10) produce the excess free energy, which can be given by the following equation:
| (20) |
Excess free energy due to the presence of sulphur is obtained considering the Henrian behaviour of the sulphides in silicate melts:
| (21) |
Here, γ°AS and γ°BS are the Henrian activity coefficients, and
| (22) |
The metallic ions (A2+ and B2+) can be placed near the free oxygen ions (O2−) or inside the silicate structure. The effect of this distribution can be obtained by following the model proposed by Dessureault and Pelton17) who suggested that in a random distribution of cations and anions in their respective sublattices, the probability that a given nearest-neighbour cation-anion pair is an A2+−O2− pair is equal to YAYO2-. However, in a non-random mixture, this probability would increase to YAYO2-+ y, where y is the probability parameter, and its value is positive. Similarly, the probability for B2+−O2− pair is equal to YBYO2- −y. Therefore, the probability of finding the group A−O−B is given by the following equation:
| (23) |
| (24) |
Here, WA,B/O is the excess free energy of the binary AO-BO system, and
| (25) |
| (26) |
For ternary systems SiO2-AO-BO, random mixing of cations A2+ and B2+ occurs in the silicate structure when the oxides within these systems behave in a similar way with silicate, which means that the SiO2-AO and SiO2-BO binary systems exhibit comparable free energies of mixing such as the SiO2–MgO–MnO and SiO2–FeO–MnO systems. However, if the AO and BO oxides exhibit strong non-random behaviours, such a SiO2–CaO–FeO, then this effect must be considered in the model. The term included in the present model is based on the similarities between the basic region (XSiO2 ≤ 1/3) of the phase diagrams of these systems and the diagrams of the reciprocal ternary systems.
The parameter representing deviation in the distribution of A2+ and B2+ from random mixing can be derived from the following equation:
| (27) |
This energy is associated with exchange energy to the following reaction at XSiO2 = 1/3:
| (28) |
The probability parameter y also represents the amount of near-neighbour pairs of A2+−O− and B2+−O2−, which transform to B2+−O− and A2+−O2−.
2.6. Non-random Mixing of A2+, B2+, O2− and O−Dessureault and Pelton17) proposed a quasichemical model of reciprocal ternary systems using a term of the configurational entropy to consider the effect of non-random mixing (Scnon-random) on the second-nearest-neighbour interactions. The following term is the approximate expression for the distribution of the A−O2−, A−O−, B−O2−, B−O− “bonds” over the z bond positions:
| (29) |
Here, z is also the coordination number.
2.7. Gibbs Energy of the Exchange ReactionThe Gibbs energy change,
| (30) |
| (31) |
Here,
Considering the silicate system, this energy term becomes the following:
| (32) |
The Gibbs free energy of mixing (Δgmix) is then obtained by combining the expressions (14), (20), (21) (24), (27), (29) and (32) through the following equation:
| (33) |
This expression is thermodynamically consistent with the Gibbs–Duhem and Gibbs–Helmholtz equations. Equation (33) reduces to the respective expressions for the binary sub-systems (SiO2-AO, SiO2-BO) or the ternary silicate system. This expression is also consistent with the A,B/O,S ‘exchange reaction’, where the molar partial free energies of the sulphides and oxides must give the following:
| (34) |
The testing of this model has been done for the ternary silicates of the SiO2–CaO–MgO–MnO–FeO system. The parameters ω and η for the binary silicate systems SiO2-MO (M = Fe, Ca, Mg, Mn) as well as the parameters of the excess free energy for the binary liquid metallic oxides AO–BO have been reported previously.18) Table 1 shows the Gibbs energy changes for the oxide/sulphide reactions at different temperatures. These thermodynamic data were taken from the FactSage database.19) Table 2 shows the values of the excess free energy due to the presence of sulphur (
| Metallic oxide | Temperature (K) | ΔG°M (J) | KM |
|---|---|---|---|
| CaO | 1773 | 61643.7 | 0.01527 |
| 1776 | 61661.2 | 0.01536 | |
| 1873 | 62447.1 | 0.01813 | |
| MgO | 1773 | 144442.0 | 5.5516×10−5 |
| 1776 | 144429.0 | 5.64922×10−5 | |
| 1873 | 144026.5 | 9.62080×10−5 | |
| MnO | 1823 | 81879.2 | 0.0045063 |
| 1873 | 81950.5 | 0.0051816 | |
| 1923 | 82014.45 | 0.0059177 | |
| FeO | 1773 | 97956.7 | 0.0013001 |
| 1776 | 97993.56 | 0.0013115 | |
| 1823 | 98592.9 | 0.0014959 | |
| 1873 | 99287.1 | 0.001702 | |
| Metallic sulphide | Temperature (K) | |
|---|---|---|
| CaS | 1773 | 3000 |
| 1776 | 3000 | |
| 1873 | 2000 | |
| MgS | 1773 | 600 |
| 1776 | 600 | |
| 1873 | −467 | |
| MnS | 1823 | 2702 |
| 1873 | 2539 | |
| 1923 | 2377 | |
| FeS | 1773 | 1500 |
| 1776 | 1500 | |
| 1823 | 1500 | |
| 1873 | 1500 |
The CS of the SiO2–CaO–MnO slag through the entire composition range was measured by Park et al.20) at the temperature of 1873 K using a gas–slag equilibration method, and by Abraham et al.21) at 1923 K. The experimental and calculated sulphide capacities of this system at 1873 K are shown in Fig. 2, where exists a good agreement except in the case of low CaO content since the Henrian activity coefficient of MnS was calculated using the results obtained by Abraham et al.21)
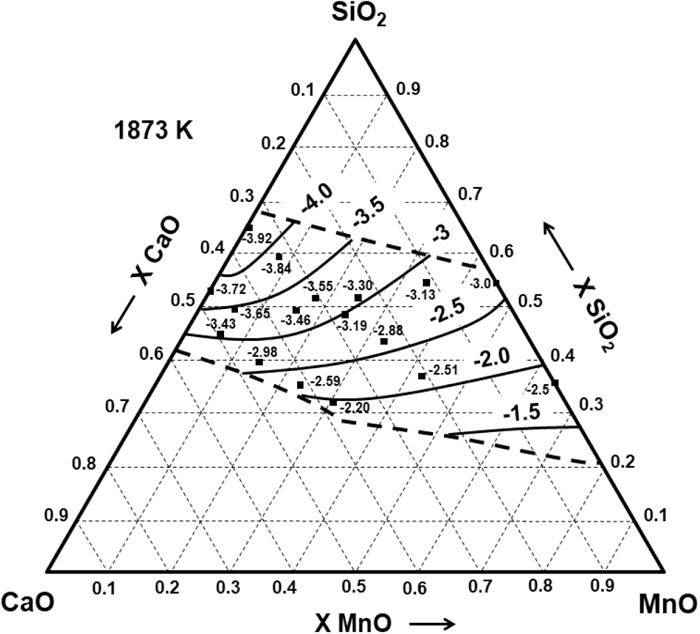
Experimental20) and calculated iso Log10(CS) curves in the SiO2–CaO–MnO system at 1873 K.
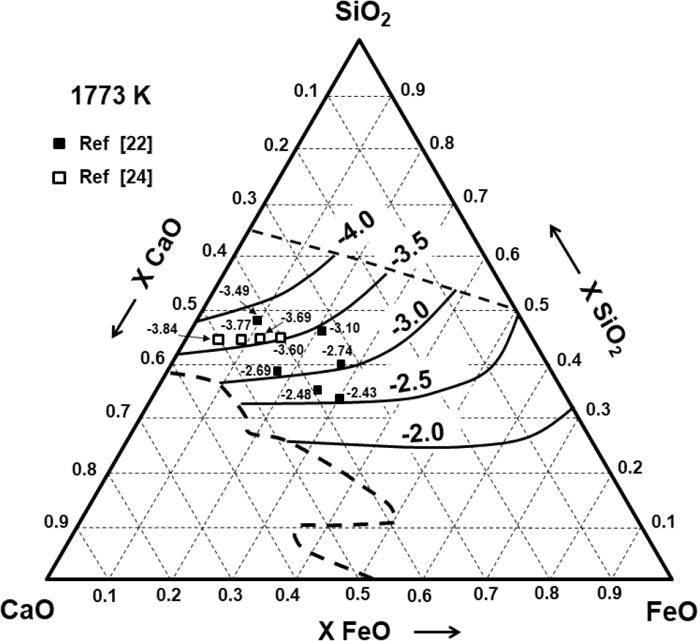
Figure 3 shows the calculated and experimentally determined values of CS in the SiO2–CaO–FeO system by Nzotta et al.22) at 1773 K using the gas-slag equilibration technique. Figure 4 shows the calculated and experimental data reported by Kalyanram et al.23) at 1773 K in the SiO2–CaO–MgO system. Bronson and St. Pierre24) also obtained the Cs for both ternary systems using simultaneous equilibration within quartz capsules at 1776 K and with XSiO2 = 0.4525, which are comparable with the calculated results in Fig. 5.
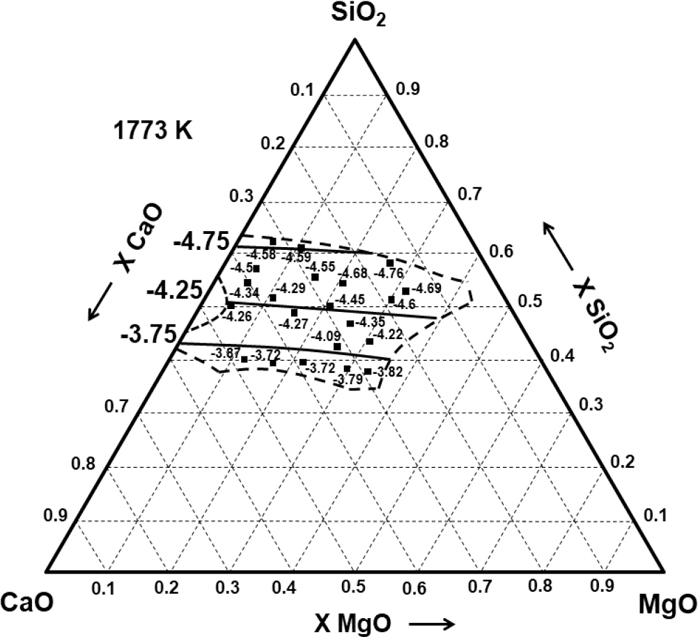
Experimental23) and calculated iso Log10(CS) curves in the SiO2–CaO–MgO system at 1773 K.
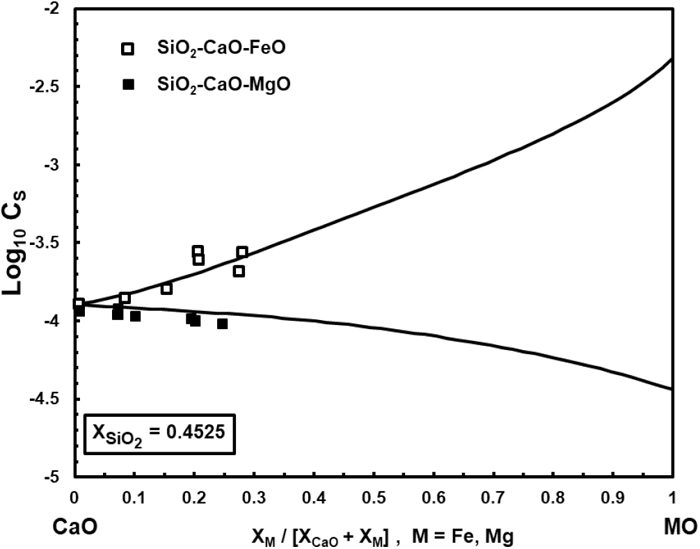
Experimental24) and calculated sulphide capacity in the SiO2–CaO–MO (M=Fe, Mg) systems at 1776 K and XSiO2 = 0.4525.
CS mainly depends on the effects of the metallic oxide as well as the relative stability of the metallic sulphide. Figure 3 demonstrates that, in the SiO2–CaO–FeO, CS increases by the addition of FeO at a fixed CaO/SiO2. Figure 5 shows the same effect when FeO substitutes CaO in a system where XSiO2 = 0.4525. These results indicate the competitiveness of CaO and FeO not only in silicate depolymerisation but also in desulphurisation reactions. Ca2+ cations are electrically balanced with two non-bridging oxygen ions (O−), indicating that the Fe2+ cations are relatively free from the role of network modifier and that they mainly participate in the desulphurisation reaction.
This can be easily explained using the energy associated with the following exchange reaction:
| (35) |
Here, Δω =
In view of the explanations given in the previous system, it is expected that CS, in the SiO2–CaO–MgO system with XSiO2 = 0.4525, increases as MgO substitutes CaO. However, in this slag, the equilibrium constants of the reactions at 1776 K are the following:
| (36) |
| (37) |
Thus, using Eq. (4) for calculating Cs for this ternary system will provide lower values when MgO substitutes CaO.
3.3. SiO2–MgO–MnO Plus SulphurFigure 6 shows the iso-sulphide capacity curves at 1923 K for SiO2–MgO–MnO system calculated by the present model. The experimental data obtained by Sharma and Richardson25) are also shown in this figure for comparison. It can be seen that the substitution of MgO by MnO at a fixed

Experimental25) and calculated iso Log10(CS) curves in the SiO2–MgO–MnO system at 1923 K.
The effect of FeO on the CS of the SiO2–MgO–FeO slags was experimentally measured at 1873 K by Kim et al.26) using the thermal chemical equilibrating technique with Fe–Ni alloys. Nzotta et al.22) also experimentally obtained the Cs for this system at 1873 K using the gas-slag equilibration technique. Figure 7 shows the calculated and experimental Cs values of this ternary system where it can be seen that the model is in good agreement with the results of Nzotta et al.22) However, there is an average difference between the calculated and the experimental results of Kim et al.26) of about Log10(CS) of 0.5.
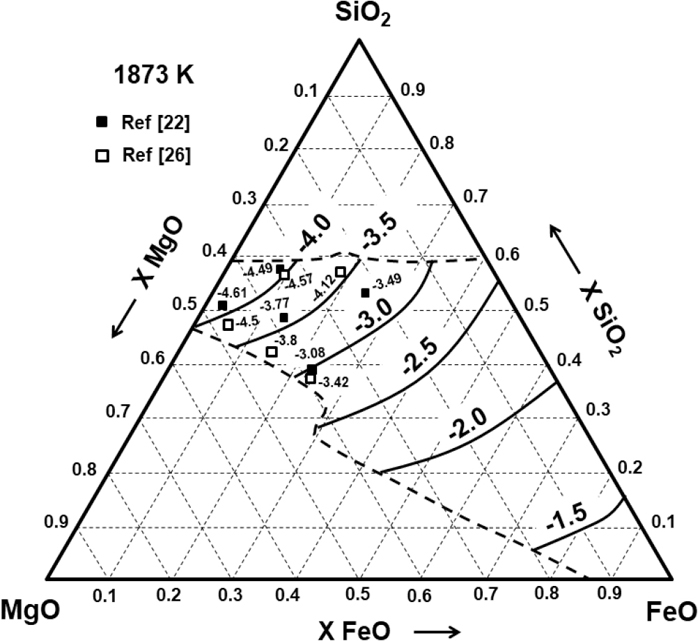
Figure 8 shows the calculated and experimental Cs results of the SiO2–FeO–MnO system obtained by Hino and Fuwa27) at 1823 K and with XFeO < 0.16. Evidently, the task of the model is to reproduce the experimental values and predict the Cs values at other temperatures and compositions as shown in Fig. 8.

Experimental27) and calculated iso Log10(CS) curves in the SiO2–FeO–MnO system at 1823 K.
Figure 9 showcases the comparison of the experimental and the calculated sulphide capacities of ternary slags in the SiO2–CaO–MgO–MnO–FeO system using the present thermodynamic-based model, where a relatively good agreement is obtained. It is worth mentioning that the model does not include any adjusted parameter for the ternary systems.

Comparisons between calculated and measured sulphide capacity of several ternary silicates of the SiO2–CaO–MgO–MnO–FeO system.
Through this study, a previous structural model13) for silicate melts and glasses is extended. It has been found that sulphide ions can be incorporated into the model, substituting quasi-lattice sites for O2−. It is assumed that each metallic oxide prompts a depolymerisation reaction O° + O2− = 2 O− with characteristic free energy change. This model can also consider the different behaviours of the metallic ions in the silicate structure. Besides, this model can calculate Cs for ternary silicates solely from the data pertaining to the binary sub-systems; no ternary terms are required, and this model is found to be thermodynamically consistent. Moreover, a good agreement was obtained between the experimental and calculated values of Cs of ternary silicates in the SiO2–CaO–MgO–MnO–FeO system.
The authors wish to thank the Institutions CONACyT, SNI, COFAA and IPN for the support of this research.