2021 Volume 61 Issue 6 Pages 1964-1970
2021 Volume 61 Issue 6 Pages 1964-1970
This study deals with austenite grain growth during high-temperature carburization of an Al- and Nb-microalloyed case-hardening steel. The grain size after carburization-simulated heating for 5 h at 1050°C decreased with the increase in the cooling rate from hot forging-simulated heating for 1 h at 1250°C. The increase in cooling rate led to the decreases in the volume fractions and sizes of AlN and Nb(C,N) particles precipitated during cooling, and AlN disappeared when the cooling rate increased to 16°C/min, while Nb(C,N) still slightly exited at 16°C/min. Because of oversaturation caused by cooling within a finite time, further precipitation occurred during the subsequent normalization for 3 h at 1070°C, resulting in the formation of AlN–Nb(C,N) combined particles. When the cooling rate increased, the volume fraction and number density of these combined particles increased while their size decreased. Therefore, a higher cooling rate causes a larger pinning effect on grain growth during carburization; thus grain size after carburization decreased with the increase in cooling rate. Transmission electron microscopy confirmed the formation of a coherent AlN–Nb(C,N) interface due to good lattice matching between the crystal planes of AlN (1120) and Nb(C,N) (220). This led to the preferential nucleation of AlN on the Nb(C,N) particles, thereby forming stable AlN–Nb(C,N) particles.
Gears and shafts made of case-hardening steel are common components in transportation equipment, and carburization applied to them improves their mechanical properties such as fatigue strength and wear resistance. The carburization process requires a considerably long time because it is based on carbon diffusion deep into the steel from the surface, especially for large components of huge construction equipment, for example. The processing time can be reduced by increasing the carburization temperature, leading to an increase in productivity and a decrease in manufacturing cost. The conventional gas carburization process typically requires 12 h at 930°C to achieve a carburization depth of 1 mm, and the processing time can be reduced to 3 h by increasing the carburization temperature to 1050°C.1) However, such high-temperature carburization can cause abnormal austenite (γ) grain growth2) and, accordingly, degrade the fatigue performance and toughness of the steel components. The abnormal grain growth can be prevented by the addition of micro-alloying elements to form pinning particles, including AlN,3,4) Nb(C,N),3,5,6,7,8,9,10,11) TiN12) and V(C,N),13) where TiN or a combination of AlN and Nb(C,N) are most commonly used in industrial applications.
In the production process of the steel components, cold or hot forging is typically used, which can influence the size and volume fraction of the pinning particles. Cold forging has recently gained popularity in the formation of small mechanical components due to its high dimensional accuracy. In cold-forged steel, it often happens that residual shear stress exists,14) spheroidizing annealing of cementite particles and dissolution of cementite,15,16) which can induce abnormal grain growth. For large components, on the other hand, hot forging is also often used, followed by normalization and carburization heat treatments. Hot forging is performed at temperatures that are high enough to dissolve precipitates, and re-precipitation occurs during the subsequent cooling and normalization steps. Therefore, the optimization of the conditions of these subsequent steps is essential to maximize the pinning effect, which requires an understanding of the precipitation behavior of each kind of precipitate. Kamada et al. investigated the effect of heat treatment involving normalization subsequent to hot forging on precipitation behavior.17) The particle size and volume fraction of the resulting AlN precipitate greatly affected the γ grain structure formed during carburization-simulated heating. Jia et al. investigated the effect of normalization conditions on abnormal grain growth behavior in Ti-microalloyed steel during carburization-simulated heating, which was conducted after solution treatment (1200°C) and quenching.18) Normalization at 650°C led to the formation of fine TiC precipitates, which effectively pinned the migration of the γ-grain boundary. However, sparsely distributed large TiC particles led to abnormal γ grain growth during annealing at 1025°C.18)
The utilization of both AlN and Nb(C,N) is feasible for the prevention of the abnormal grain growth at high-temperature carburization because they have high volume fraction and Nb(C,N) has higher dissolution temperature. The effect of hot-forging temperature on the γ-grain structure in Al- and Nb-microalloyed case-hardening steel has been already reported by the present authors.19) A combination of high-temperature hot-forging and subsequent slow furnace cooling caused the coarsening of the γ-grain during a high-temperature carburization. This was attributed to the dissolution of AlN and Nb(C,N) particles, and the re-precipitation and growth of these particles during slow furnace cooling. The previous study19) found that the cooling rate after hot-forging affected precipitates. Although the individual effect of cooling rate on Al- or Nb-microalloyed steels has been reported by many researchers,17,20,21) the same effect for Al- and Nb-microalloyed case-hardening steels has not yet been fully understood. Particularly, the origin of the AlN–Nb(C,N) combined particles formation continues to be unclear. Understanding the precipitation behaviors of AlN and Nb(C,N) particle will contribute to the control of the precipitate and optimization of industrial manufacturing processes to suppress abnormal grain growth. Therefore, this study investigated the effects of cooling rate after hot forging on the precipitation behavior of these particles and grain structure formed at a high-temperature carburization. The precipitates and γ-grain structure, as well as the interfacial structures of AlN and Nb(C,N) were analyzed. Further, the formation mechanism of AlN–Nb(C,N) combined particles was explored.
The Al- and Nb-microalloyed JIS SCM420-based case-hardening steel, namely Fe-0.20C-0.26Si-0.85Mn-0.015P-0.99Cr-0.16Mo-0.035Al-0.032Nb-0.007Ti-0.0250N alloy (mass%), was prepared via 20 kg vacuum induction melting and hot-rolling to form a rod with a diameter of 30 mm. Small samples of the as-received steel were forging-simulated heated at 1250°C for 1 h, followed by cooling under different conditions/rates, namely water quenching, and furnace cooling at 16, 4, and 0.7°C/min (Fig. 1). The samples were heated at 50°C/min, normalized at 1070°C for 3 h and furnace cooled at 16°C/min. The sample was then carburization-simulated heated to 1050°C for 5 h and cooled via water quenching. The heat-treated samples were sectioned in the transverse direction, mechanically polished, and etched using a 4 mass% picric acid aqueous solution at 50°C to observe the γ-grain structure using an optical microscope (VHX-6000, KEYENCE Corp.).

Heating pattern of the Al- and Nb-microalloyed case-hardening steel samples, including forging-simulated heating, cooling via water quenching (WQ) or furnace cooling (FC), normalization, cooling via FC, carburization-simulated heating, and cooling via WQ.
The precipitates were observed using scanning transmission electron microscopy (STEM; Titan3 G2 60-300, FEI Company) to evaluate the effect of different cooling treatments after forging-simulated heating on the precipitation behavior during normalization. The STEM samples were prepared by sandwiching a plate-like sample (<0.5 mm thickness) between two Mo plates using epoxy resin. The Mo plate reduced the volume of the steel sample, thereby reducing the effect of the magnetic force in the STEM chamber. The sandwiched sample was sliced into thin films, which were placed on a single-hole Mo grid. The samples were polished to reduce the thickness below 20 μm, and ion-milled with 5 kV Ar ions using a precision ion polishing system (PIPS II, model 695, Gatan Inc.). Because it was difficult to distinguish the Nb(C,N) particles from Fe matrix using high-angle annular dark field (HAADF) imaging, energy dispersive spectroscopy (EDS) mapping was used instead to analyze the precipitates. A high probe current (~6 nA) was applied during STEM-EDS mapping, where the pixel size was 2 or 4 nm. Each sample treated under each cooling condition/rate was analyzed at a total of 30 positions. The volume fraction of the precipitates was measured by quantifying the sample thickness of each observed area using electron energy loss spectroscopy.22)
The precipitate was observed using the extraction replica method.23) The steel was electrically etched in an acetylacetone (10%) and tetramethylammonium (1%) methanol solution, and the exposed precipitates on the sample surface were transferred into a replica film. Carbon was deposited onto the film. The film was dissolved in acetone, leaving a carbon sheet with the precipitates, which was captured on a copper grid. The precipitates on the carbon film were observed using STEM. The precipitates were collected using a 0.2 μm filter (Millipore), and their crystal structure was analyzed using X-ray diffraction (XRD) (Rigaku MiniFlex600). Thermodynamic calculations were performed using the Thermo-Calc software (version 2019) and the TCFE9 database.24)
γ-grain structure was observed in the samples quenched after carburization-simulated heating. The heating temperature and time of the carburization-simulated heating was similar, but the grain size was different, as shown in Fig. 2. The γ-grain size was strongly affected by the cooling rate after the forging-simulated heating, which was performed before the normalization and carburization-simulated heating. Generally, the difference in grain size can be attributed to the difference in the distribution of the pining particles, when the temperature and duration of the grain growth is similar. Precipitates such as AlN and Nb(C,N) exist in the present Al- and Nb-microalloyed steel, thus the size and volume fraction of these precipitates can affect the γ-grain structure.19,21) Therefore, the relationship between the cooling rate and the distribution of pinning particles was investigated.
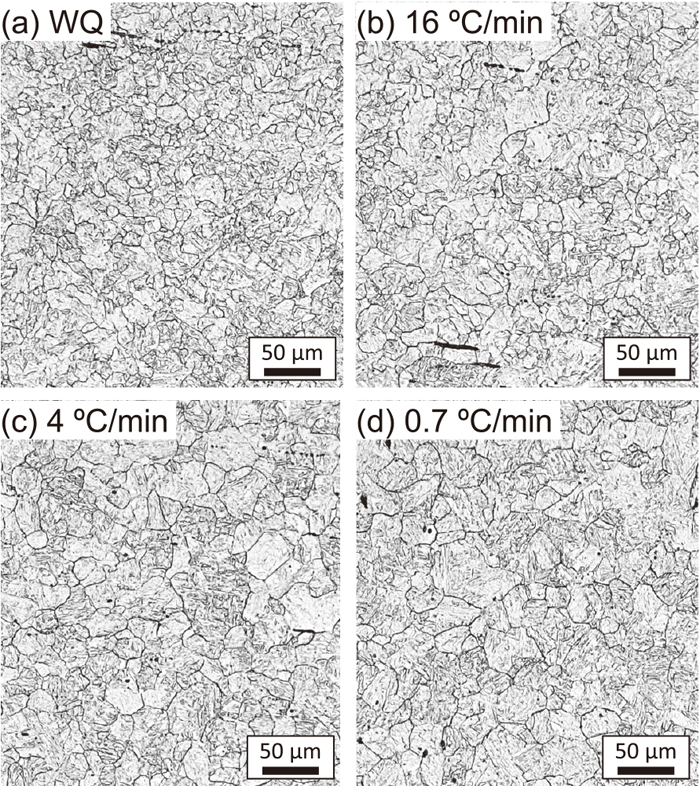
Optical micrographs showing the effect of the cooling rate after the forging-simulated heating on the γ-grain structure of the samples after the carburization-simulated heating.
STEM-EDS maps of precipitates are shown in Fig. 3. The four maps correspond to the four different rates of cooling from the forging-simulated heating. The EDS map of the water-quenched sample exhibited fine TiN particles without AlN and Nb(C,N) particles (Fig. 3(a)). The calculated dissolution temperatures of AlN, Nb(C,N), and TiN are 1198, 1218, and 1468°C, respectively, according to thermodynamic calculations shown in Fig. 4. Therefore, the AlN and Nb(C,N) particles within the as-received steel dissolved completely during the forging-simulated heating at 1250°C, and could not precipitate due to quenching, while TiN particles did not dissolve during heating and existed in the quenched sample (Fig. 3(a)). When cooled at 16°C/min, a small number of Nb(C,N) particles precipitated during cooling, although AlN particles were not observed yet (Fig. 3(b)). The TiN particles in this sample also contained traces of Nb and C, while the Nb(C,N) particles contained a small amount of Ti. Some Nb(C,N) particles had precipitated on the TiN particles as a nucleate site during cooling, resulting in the formation of TiN–Nb(C,N) combined particles as shown in the inset image in Fig. 3(b). Because both TiN and Nb(C,N) particles have NaCl-type crystal structure, the Nb(C,N) may be precipitated to the TiN particles due to the formation of stable semi-coherent interface. When the cooling rate was reduced to 4°C/min, the number of Nb(C,N) precipitates increased (Fig. 3(c)), and very few AlN particles were observed in certain other observation fields. At the lowest cooling rate of 0.7°C/min, the number and size of AlN particles are large, and some of them were combined with Nb(C,N) particles (Fig. 3(d)).

STEM-EDS maps of precipitates in samples cooled at different rates after forging-simulated heating. Inset figure in (b) shows TEM image of TiN–Nb(C,N) combined particles acquired using a replica method. (Online version in color.)

Relation between the volume fraction of each precipitate and temperature, determined using thermodynamic calculations. (Online version in color.)
These results demonstrated that the precipitation rate of AlN was lower than that of Nb(C,N), which was also previously reported by Kubota and Ochi.1) They investigated the precipitation behaviors of AlN and Nb(C,N) in case-hardening steels at different cooling rates (3 to 3000°C/min) using fully automated transformation measuring equipment (Formaster). The volume fractions of AlN and Nb(C,N) precipitates found in the samples cooled from 1000°C at 3°C/min were lower than those in the sample quenched after 2 h-holding at 900°C, and the ratios of the former to the latter were 7% and 82%, respectively. Other studies have also reported the slow precipitation of AlN during cooling after solution treatment.25) On the other hand, it is reported that the precipitation of AlN during heating of oversaturated steel is faster than it is during the cooling of solution-treated steel.26) These phenomena may be attributed to differences in the crystal orientation relationships between the matrix (γ or α-ferrite) and precipitates such as AlN and Nb(C,N). It is reported that γ-matrix and Nb(C,N) particles have coherent or semi-coherent interfaces with a cube–cube orientation relationship, as shown by [110]NbC//[110]γ, (111)NbC//(111)γ.27) A low interfacial energy of such an orientation relationship may enhance the precipitation of Nb(C,N) particles. In contrast, it is reported that the volumetric misfit between the AlN and the steel matrix is high, thus the precipitation of AlN in γ-matrix occurs predominantly at grain boundaries.28,29)
Other STEM-EDS maps obtained for the normalized samples revealed the formation of a large number of fine AlN–Nb(C,N) combined particles in the sample that was water-quenched from the forging-simulated heating and then normalized (Fig. 5(a)). In this water-quenched sample, Al and Nb were oversaturated in the matrix phase before normalization because AlN and Nb(C,N) particles were not precipitated (Fig. 3(a)). This facilitated the nucleation of AlN and Nb(C,N) and formation of fine precipitates during normalization. When the Al and Nb oversaturated steels are heated, precipitations of AlN and Nb(C,N) begin at approximately 600–700°C during heating.1,30) Thus, possible precipitation nucleation sites include the γ-grain boundary, martensite lath boundary, precipitated carbide on the lath boundary, and dislocations in the matrix introduced by water quenching. The possibility of nucleation sites of these precipitates should be investigated further. The most rapid furnace cooling (16°C/min) led to the formation of slightly larger AlN–Nb(C,N) combined particles (Fig. 5(b)), and lower cooling rates caused further coarsening (Figs. 5(c) and 5(d)). Slower cooling allowed for initial Nb(C,N) particle precipitation and prolonged growth to form coarser particles. Coarse AlN was predominantly nucleated on these coarse Nb(C,N) particles.

STEM-EDS maps of precipitates in samples after normalization, that was performed after cooling from forging-simulated heating under different conditions, namely (a) water quenching, and furnace cooling at (b) 16, (c) 4, and (d) 0.7°C/min. (Online version in color.)
The volume fractions and particle diameters of AlN, TiN, and Nb(C,N) were analyzed from STEM-EDS maps; the results are summarized in Figs. 6 and 7. The Nb(C,N) particles were categorized as either isolated ones or the ones combined with AlN particles, while the isolated AlN particles and AlN–Nb(C,N) combined particles were considered to be a single category. The AlN particles have a plate-like morphology, which caused difficulties in the measurement of thickness and diameter of the particle from a single STEM image. Therefore, the diameter of the AlN and AlN–Nb(C,N) combined particle was determined by measuring two diagonal lines, where a spherical shape was assumed. The obtained values of the volume fraction and particle diameter were associated with a measurement error, as the data was derived from 30 EDS maps from a limited measurement area. The accuracy of the measurements was dependent on the number of measured precipitate particles. Thus, it should be noted that samples with a small number of coarse precipitate particles (e.g. those in the sample cooled at 0.7°C/min) led to fewer individual AlN particle measurements, resulting in a larger error.

Volume fractions of each precipitate (a) after forging-simulated heating and (b) after normalization. (Online version in color.)

Diameters of the AlN particles (including AlN–Nb(C,N) combined particles), isolated Nb(C,N) particles and isolated TiN particles measured after (a) the forging-simulated heating and (b) the subsequent normalization. (Online version in color.)
The thermodynamic calculations revealed that volume fractions of AlN, Nb(C,N), and TiN at 1070°C are 0.15%, 0.06%, and 0.03%, respectively. AlN particles are not fully precipitated after forging-simulated heating even in the case of the slowest cooling at 0.7°C/min, as shown in Fig. 6(a). However, Nb(C,N) particles are fully precipitated at a higher cooling rate of 4°C/min. These results demonstrate that the precipitation rate of Nb(C,N) is higher than that of AlN. The volume fraction of each precipitate significantly increased after normalization (Fig. 6(b)). The ratio of AlN–Nb(C,N) combined particles increased after normalization, implying that Nb(C,N) particles played the role of AlN nucleation sites. The AlN–Nb(C,N) combined particles were the main pinning particles of the γ-grains, while the isolated Nb(C,N) and TiN particles had minimal pinning effect due to their extremely small volume fraction.
The particle diameter of TiN was ~50 nm under any given cooling condition, while the isolated Nb(C,N) particle diameter varied slightly from 50 to 70 nm. However, the particle diameter of AlN (including AlN–Nb(C,N) combined particles) after normalization was highly dependent on the cooling rate after the forging-simulated heating. When the sample was cooled rapidly by water quenching, fine AlN–Nb(C,N) combined particles precipitated during normalization. However, lower cooling rates led to coarse precipitation of Nb(C,N) during cooling, which triggered coarse AlN precipitation during the subsequent normalization step. The AlN particle size affected the γ-grain structure (Fig. 2). The relationship between grain size and pinning particle behavior can be described using the Zener equation:5,30)
| (1) |
The γ grain diameter was estimated from the measured particle diameter (Fig. 7) and the volume fraction (Fig. 6) using the Zener equation (Eq. (1)) with the constant K = 4/3. In the case of this study, the average radius of each kind of precipitate is quite different from each other. Therefore, the pinning forces ΔPpin of each kind of precipitate was calculated using Eq. (2).19)
| (2) |
| WQ | 16°C/min | 4°C/min | 0.7°C/min | |
|---|---|---|---|---|
| Observed grain diameter (μm) | 23 | 29 | 33 | 44 |
| Estimated grain diameter (μm) | 15 | 20 | 33 | 47 |
Co-doping of steel with Al and Nb leads primarily to the formation of AlN–Nb(C,N) combined particles, where the initially precipitated Nb(C,N) play the role of nucleation sites for subsequent AlN precipitation. In order to investigate the interface between AlN and Nb(C,N), STEM was performed. Figure 8 shows a HAADF-STEM image of the AlN–Nb(C,N) combined particles found in the normalized sample cooled at 0.7°C/min after the forging-simulated heating, where the precipitate particles were collected via the extraction replica method. Figure 8(a) shows the HAADF-STEM image of the AlN–Nb(C,N) combined particles, and Figs. 8(b), 8(c) show side-viewed STEM-EDS maps of these combined particles. These results reveal that the Nb(C,N) particles with an elongated hemispherical morphology are attached to the surface of faceted plate-like AlN particles. The morphology of these AlN–Nb(C,N) combined particles suggests that AlN–Nb(C,N) has a low interface energy.

(a) HAADF-STEM image and (b, c) STEM-EDS maps of the AlN–Nb(C,N) combined particles in the normalized samples cooled at a rate of 0.7°C/min after forging-simulated heating. The precipitate particles were collected via the extraction replica method. (Online version in color.)
The high-resolution HAADF-STEM images of the AlN–Nb(C,N) interface demonstrated that AlN had a wurtzite-type single crystalline structure with a plate-like surface consisting of (0001) planes (Fig. 9(a)). The Nb(C,N) particles also exhibited a single crystalline NaCl-type structure, where the (111) plane of the Nb(C,N) particle was parallel to the AlN (0001) plane. The lattice of the AlN (1120) plane matched well with the Nb(C,N) (220) plane. Thus, a coherent AlN–Nb(C,N) interface was formed. XRD of the collected precipitate via electrolysis indicated that the lattice distance was 0.1557 nm in the AlN (1120) plane, and 0.1575 nm in the Nb(C,N) (220) plane. This good lattice matching facilitated the formation of stable AlN–Nb(C,N) combined particles with a low interfacial energy. The AlN (0001) plane is a polar interface due to the presence of Al-polar and N-polar planes. Although the location of the nitrogen atoms was unclear both in the enlarged HAADF-STEM image (Fig. 9(b)) and the corresponding atomic structures (Fig. 9(c)), the coherency of the AlN/Nb(C,N) interface does not seem to be strongly dependent on the polarity of AlN because Nb(C,N) particles sometimes attached to both sides of an AlN particle (Fig. 8(c)). The formation of a similar coherent interface was reported in wurtzite-type AlN–TiN layers fabricated via physical vapor deposition.32,33) Due to good lattice matching, the Nb(C,N) particles played a role of nucleation sites for AlN. To reduce the size of the pinning particles in the Al- and Nb-microalloyed steel, the reduction in the size or the increase in the number of Nb(C,N) particles is required. When the steel contains plural micro-alloying elements and multiple precipitates are formed, the precipitation rate of each precipitate, the lattice matching between the two different precipitates, and the crystal–structure relationship between precipitate and matrix are all important factors for determining the morphology and distribution of the precipitate particles.

(a, d) High-resolution HAADF-STEM images, (b, e) enlarged images, and (c, f) corresponding atomic structures of the interface between AlN and Nb(C,N) at different incident directions. (Online version in color.)
In Al- and Nb-microalloyed case-hardening steel (JIS SCM420), the cooling rate after hot forging was found to affect the particle size of precipitates formed during the subsequent normalization and, thereby, the γ-grain structures formed during the subsequent carburization-simulated heating. Rapid cooling via water quenching ensured the complete dissolution of AlN and Nb(C,N) in the matrix (i.e. prevented precipitation). Consequently, fine AlN–Nb(C,N) combined particles formed during normalization due to the oversaturated conditions at low temperatures. The distribution of the fine particles led to a refined γ-grain structure formed during a carburization-simulated heating. Slower cooling after forging-simulated heating led to the precipitation of Nb(C,N) particles, which continued to grow as the temperature decreased. The precipitated Nb(C,N) particles played a role of nucleation sites for the coarse AlN particles during cooling and normalization. The coarse AlN–Nb(C,N) combined particles, formed as a result of slow cooling, led to a low pinning force of the precipitate, thereby leading to a coarse γ-grain structure after carburization-simulated heating. Atomic-resolution observation of the AlN–Nb(C,N) interface indicated that the AlN (1120) plane was well-matched with the Nb(C,N) (220) plane, thereby facilitating the formation of a coherent interface. Thus, stable AlN–Nb(C,N) combined particles with low interfacial energies were readily formed. Overall, rapid cooling after solution treatment effectively promoted grain refinement in Al- and Nb-microalloyed case-hardening steel. These findings provide insight to control the microstructure of steel.
The authors would like to thank Dr. Eng. Mr. Tianlong Zhang, M. Eng. Ms. Yukiko Tanaka and M. MedSc. Ms. Ami Matsuno for their extensive experimental support. We are grateful to Mr. Kenji Ohkubo, Mr. Ryo Ota, Mr. Takashi Tanioka and Ms. Emiko Obari for their technical assistance with the TEM. Part of this work was conducted at the Hokkaido University, supported by the “Nanotechnology Platform” Program of the Ministry of Education, Culture, Sports, Science, and Technology (MEXT), Japan. This work was supported by Grant-in-Aid for Young Scientists (No. 20K15055).