2021 Volume 61 Issue 7 Pages 2181-2184
2021 Volume 61 Issue 7 Pages 2181-2184
This paper suggested an improved method for the sample preparation of pig iron and cast iron in inductively coupled plasma atomic emission spectrometry. Conventional digestion methods using a mixture of hydrochloric acid and nitric acid, associated with fuming with phosphoric and sulfuric acids or fuming with perchloric acid, have a poor-ability to decompose the iron sample completely. Alternatively, a microwave digestion method using an acidic mixture of hydrofluoric acid, nitric acid, phosphoric acid, and potassium chlorate, enabled various iron samples to be fully decomposed. The suggested procedure was applicable to quantify titanium, vanadium, chromium, manganese, nickel, copper, and molybdenum in high-carbon iron samples using a similar dissolution method.
Chemical analysis of pig iron should be conducted with high accuracy for the quality control of the steel manufacturing. In general, spark discharge atomic emission spectrometry and X-ray fluorescence spectrometry are employed for this purpose.1) Obtained signals of the emission intensity and X-ray fluorescence intensity depend not only on the chemical composition but also on the metallic texture and the history of thermal treatment. Therefore, these analytical methods require appropriate reference materials to calibrate analytical values. In-house standards, which should be carefully analyzed by using a wet chemical analysis, have been frequently used because of lack of commercial reference materials. Additionally, the chemical composition of several metal constituents is also important for commercially producing pig iron as an intermediate raw material in the steel-making process.
Although metal constituents such as titanium and chromium, which may form inclusions in pig iron, need to be quantified for the quality control, inductively coupled plasma atomic emission spectrometry (ICP-AES) cannot be applied for the quantification of the iron sample including pig iron and cast iron on the basis of the Japanese Industrial Standards (JIS).2) In addition, the decomposition of an iron sample in a high-content of carbon should be carefully conducted. Chromium carbide would remain after fuming with phosphoric and sulfuric acids and when adding nitric acid as an oxidizing agent in the fumed solution.3) Such a carbide can be completely decomposed by adding potassium permanganate as an oxidizing agent. However, this oxidization method cannot be used for multi-elemental ICP-AES analysis including manganese and chromium. On the other hand, the JIS method for the atomic absorption spectrometric determination of titanium adopts a solution preparation based on the fuming with perchloric acid.4) However, this treatment might evaporate chromium from the sample solution.
An alternative procedure having merits for the efficiency and simplicity is the application of microwave-assisted digestion. This digestion method has been employed to completely decompose steel samples5,6) and metal carbides and oxides in a steel samples.7) Our previous paper8) has described an analysis of various kinds of steel samples in which a microwave digestion method is conducted for preparing the sample solution in ICP-AES. An acid mixture of hydrochloric acid, hydrofluoric acid, nitric acid and phosphoric acid was applicable to digest tool steel, high-speed steel, and stainless steel. However, this acid mixture has not been tested to prepare sample solution for pig iron and cast iron. This paper suggests a sample preparation method by microwave-assisted digestion with the oxidative acid mixture, in the quantification of titanium, vanadium, chromium, manganese, nickel, copper, and molybdenum by ICP-AES.
A microwave oven of TOPwave® (Analytik Jena AG, Jena, Germany) was used for the digestion of an iron sample. The digestion procedure was conducted in a 100 cm3 of polytetrafluoroethylene vessel. A spectrometer of Arcos® (SPECTRO Analytical Instruments GmbH, Kleve, Germany) was used for the quantification of alloying elements in the iron sample. The analytical conditions for the ICP-AES analysis were listed in Table 1.
| Radio frequency of generator/MHz | 27.12 | |
| Power of generator/kW | 1.20 | |
| Flow rate of argon gas/dm3 min−1 | Coolant gas | 13.0 |
| Auxiliary gas | 1.00 | |
| Nebulizer gas | 0.65 | |
| Nebulizer | Concentric type | |
| Spray chamber | Polytetrafluoroethylene cyclone type | |
| Peristaltic pump rate | 15 rounds per minute | |
| Plasma observation | Radially | |
| Accumulation time/s | 10 |
Several acid mixtures for the iron digestion (Table 2) were prepared with the following acid reagents: 50-mass% hydrofluoric acid (electronic grade; Morita Chemical Industries Co., Ltd., Osaka, Japan), 30-mass% hydrogen peroxide (guaranteed reagent; FUJIFILM Wako Pure Chemical Corp., Osaka, Japan), and acid reagents for electronics industry (FUJIFILM Wako Pure Chemical Corp., Osaka, Japan) of 70-mass% nitric acid, 85-mass% phosphoric acid, 35-mass% hydrochloric acid, and 96-mass% sulfuric acid. Potassium chlorate (guaranteed reagent; FUJIFILM Wako Pure Chemical Corp., Osaka, Japan) was used as an oxidizing agent.
| Test | Acidic mixture on first heating | Additional reagent | Tested iron sample | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Volume ratio of acidic reagent | KClO3 as oxidizing agent | On second heating | On third heating | Pig iron for steel making (JSS 102-5) | Cast iron (JSS 120-1) | Pig iron for casting (JSS 110-10) | ||||||
| HNO3 | HF | H3PO4 | HCl | H2SO4 | H2O2 | |||||||
| A | 1 | 1 | 1 | 1 | R(s) | R(l) | ||||||
| B | 1 | 1 | Precipi. | Precipi. | ||||||||
| C | 1 | 1 | R(s) | Sam. | ||||||||
| D | 1 | 1 | Precipi. | Precipi. | ||||||||
| E | 2 | 1 | 1 | R(s) | Sam. | |||||||
| F | 1 | 1 | 1 | R(l) | R(l) | |||||||
| G | 1 | 1 | 1 | 1 | R(s) | R(l) | ||||||
| H | 1 | 1 | 1 | 1 | R(s) | R(l) | ||||||
| I | 1 | 1 | 1 | 1 | Explo. | Explo. | ||||||
| J | 1 | 1 | 1 | 1 | 1 | R(l) | Sam. | |||||
| K | 4 | 1 | 4 | 1 | R(l) | R(l) | ||||||
| L | 4 | 1 | 2 | 1 | 2 | R(l) | R(l) | |||||
| M | 4 | 1 | 1 | 4 | R(l) | Sam. | ||||||
| N | 1 | 1 | 1 | 1 | 1.0 g | Explo. | Explo. | |||||
| O | 1 | 1 | 1 | 1.0 g | S | R(s) | ||||||
| P | 1 | 1 | 1 | 1 | 1.0 g | Explo. | Explo. | |||||
| Q | 1 | 1 | 1 | 1.0 g | 1.0 g of KClO3 | S | S | R(s) | ||||
| R | 1 | 1 | 1 | 2.0 g | S | R(l) | R(l) | |||||
| S | 1 | 1 | 1 | 1.0 g | 1.0 g of KClO3 | Precipi. | Precipi. | Precipi. | ||||
| T | 1 | 1 | 1 | 1.0 g | 2.0 cm3 of HNO3 and 1.0 g of KClO3 | S | S | R(s) | ||||
| U | 1 | 1 | 1 | 1.0 g | 2.0 cm3 of H2O2 and 1.0 g of KClO3 | S | R(l) | R(l) | ||||
| V | 1 | 1 | 1 | 1.0 g | 2.0 cm3 of H2O2 | 1.0 g of KClO3 | S | S | (S) | |||
Test sample was treated with 10 cm3 of mixed acid by using a microwave oven with the heating program of (1) as described in section 3.1.
S: successfully digested; (S): successfully digested with 5-steps heating described in section 3.1.; R(s): small amount of residue; R(l): large amount of residue; Explo.: explosive reaction (banging and/or sample loss) on adding mixed acid to sample; Sam.: undigested sample; and Precipi.: poorly-soluble precipitate with residue and/or undigested sample.
The calibration standard solution was obtained by using metal standard solutions prepared with pure metals of titanium, vanadium, chromium, manganese, nickel, copper, and molybdenum. Pure iron (99.99 mass%, MAIRON® SHP; Toho Zinc Co., Ltd., Tokyo, Japan) as the matrix element was digested in a similar manner to high-carbon iron samples and the metal standard solutions of each analyte were added to the resulting iron solution to obtain their calibration curves. Seven calibration standard solutions containing each analyte over 10 to 20-times concentration ranges were prepared to carry out the interpolation calibration.
2.3. Reference MaterialsSeveral certified reference materials (CRMs), which were purchased from The Japan Iron and Steel Federation (Tokyo, Japan), were used to validate the present analytical procedure: JSS 102-5 of pig iron for steel making, JSS 110-10 of pig iron for casting, and JSS 120-1 of cast iron.
Our previous study reported that a quaternary acid mixture of HCl, HF, HNO3, and H3PO4 was applicable to the decomposition of various kinds of steel samples including tool steel and stainless steel.8) However, high-carbon iron such as pig iron and cast iron cannot be digested with this acid mixture completely (test A in Table 2). Therefore, the digestion tests with various kinds of mixed acid were conducted as shown in Table 2. Three quaternary acid compositions of HF, HNO3, and H3PO4 mixed with HCl, H2SO4, or H2O2 (test A, G, and H in Table 2) were superior to the others. These mixtures were more oxidative than HNO3. To digest black-colored residue which was carbon and/or any carbon compound like carbide, as shown in Fig. 1, potassium chlorate was added as an oxidizing agent (e.g., test O in Table 2). Furthermore, the sample could be discomposed more effectively when hydrogen peroxide was added before the heating treatment with potassium chlorate (test V in Table 2) rather than when the amount of potassium chlorate increased or it was added repeatedly (test Q in Table 2).

Examples of the resulting solution after microwave heating of an iron sample. (a) small amount of residue on the test A in pig iron of JSS 102-5, (b) large amount of residue on the test A in cast iron of JSS 120-1, (c) poorly-soluble precipitate on the test S in pig iron of JSS 110-10, and (d) undigested sample on the test E in JSS 120-1. The solution was transferred to a 52-mm diameter vessel for the confirmation of residues.
A flow chart of Fig. 2 indicates the digestion procedure for pig and cast iron samples, which was determined from the result of the test decompositions as described above. The microwave oven was operated using a three-step heating program as follows: (1) increasing temperature to 473 K7) in 15 min and keeping the temperature for 30 min, (2) increasing temperature to 453 K in 15 min and keeping the temperature for 15 min, and (3) increasing temperature to 493 K in 15 min and keeping the temperature for 15 min. The heating program (2) was employed to avoid a drastic reaction between potassium chlorate as the oxidizing agent and hydrogen peroxide, which was added to promote the oxidization reaction. The simultaneous addition of these reagents (e.g., test U in Table 2) had no synergy effect to digest the residue. This was because potassium chlorate and hydrogen peroxide immediately reacted with each other before their digestions. The mild heating condition of the program (2) prevented a large loss of hydrogen peroxide. On the other hand, the heating program (3) of higher temperature than the program (1) was used to decompose the residue completely. The iron sample (about 0.10 g) was weighed and placed in a digestion vessel, together with an acid mixture (10 cm3) of hydrofluoric acid, nitric acid and phosphoric acid with 1:1:1 volume ratio and 0.50 g of potassium chlorate. The digesting vessel was closed with a polytetrafluoroethylene lid. The vessel was heated via the heating program (1) and then cooled down to room temperature. 2.0 cm3 of hydrogen peroxide was added to the reaction product and the vessel was subsequently heated via the heating program (2). After cooling, 1.50 g of potassium chlorate was further added in the vessel and it was heated via the heating program (3). When several samples were not decomposed completely (e.g. JSS 110-10 in the test V in Table 2), the heating procedures with adding hydrogen peroxide (1.0 cm3) and potassium chlorate (0.75 g) were repeated, as shown in the flow chart in Fig. 2. The resulting specimen solutions contained the same amounts of reagents regardless of the repeated treatments.

Flow chart of procedure of sample preparation.
An effect of the addition of potassium chlorate was tested with a synthetic specimen solution containing iron and analytes, whose concentrations were 1.0 μg cm−3 of Ti,V, Cr, Ni, Cu and Mo, and 5.0 μg cm−3 of Mn. Their emission intensities were little affected by the addition of potassium chlorate (Fig. 3). The amount of potassium chlorate was fixed to be 2.0 g in the present analysis, because the larger amount of potassium chlorate than 2.0 g could not be dissolved in 10 cm3 of the digestion acid mixture.
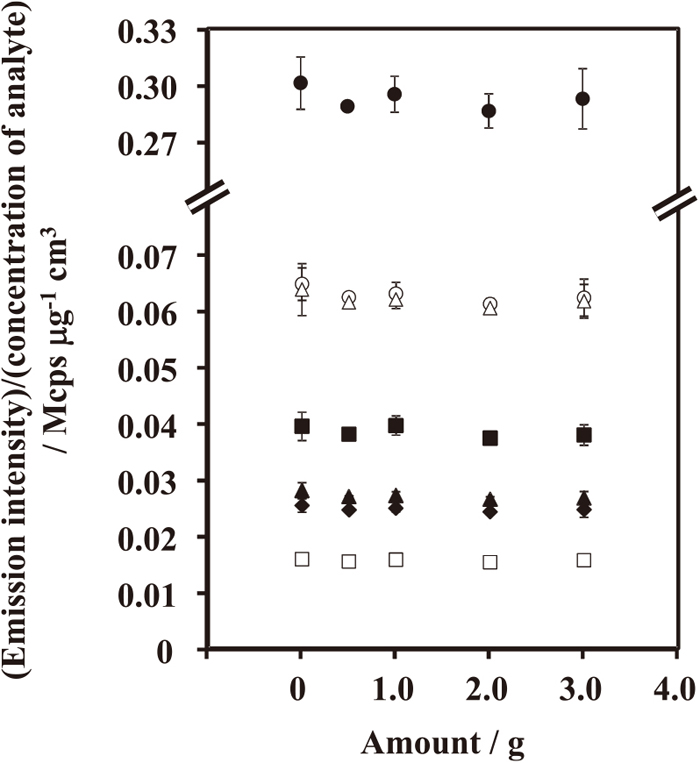
Variations in the sensitivities of emission lines with the amount of potassium chlorate as an oxidizing agent in a synthetic specimen solution. Titanium 336.121 nm (●), vanadium 292.464 nm (○), chromium 284.984 nm (■), manganese 403.076 nm (□), nickel 221.648 nm (▲), copper 327.396 nm (△), and molybdenum 281.615 nm (◆).
Calibration standard solutions containing analyzed elements (Ti, V, Cr, Mn, Ni, Cu, and Mo) and matrix iron (0.10 g) were prepared to obtain calibration curves for the ICP-AES quantification. The present calibration ranges were as follows: (0.001 to 0.38) mass% for titanium 336.121 nm, (0.002 to 0.86) mass% for vanadium 292.464 nm, (0.001 to 0.38) mass% for chromium 284.984 nm, (0.01 to 2.0) mass% for manganese 403.076 nm, (0.002 to 0.85) mass% for nickel 221.648 nm, (0.001 to 0.18) mass% for copper 327.396 nm, and (0.002 to 0.44) mass% for molybdenum 281.615 nm. Their lowest values were estimated from the lower limit of quantification, which was determined by 10-times as much as the standard deviation of blank measurements. Their highest values were calculated by the highest concentration value of the prepared calibration standard solutions. Several metallic elements in the iron CRMs were quantified by the present method as shown in Table 3. Analytical values well agreed with each certified value.
| JSS 102-5 (pig iron for steel making) | JSS 110-10 (pig iron for casting) | JSS 120-1 (cast iron) | ||||
|---|---|---|---|---|---|---|
| Analytical value | Certified value | Analytical value | Certified value | Analytical value | Certified value | |
| Ti | 0.058 ± 0.001 | 0.057 | 0.053 ± 0.001 | 0.055 | 0.0066 ± 0.0005 | 0.006 |
| V | 0.011 ± 0.001 | – | 0.0086 ± 0.0009 | 0.009 | 0.0073 ± 0.0010 | 0.007 |
| Cr | 0.019 ± 0.001 | – | 0.020 ± 0.001 | 0.019 | 0.284 ± 0.005 | 0.28 |
| Mn | 0.302 ± 0.009 | 0.30 | 0.383 ± 0.008 | 0.39 | 0.717 ± 0.012 | 0.70 |
| Ni | 0.0024 ± 0.0005 | – | 0.014 ± 0.002 | 0.013 | 0.112 ± 0.001 | 0.12 |
| Cu | <0.003 | – | 0.0048 ± 0.0014 | 0.004 | 0.299 ± 0.004 | 0.29 |
| Mo | <0.004 | – | <0.004 | – | 0.0066 ± 0.0024 | 0.007 |
Sample solutions of pig iron and cast iron could be prepared by using microwave-assisted digestion through a repeated heating and cooling procedure, using an acid solution of hydrofluoric acid, nitric acid, and phosphoric acid with 1:1:1 volume ratio and potassium chlorate as an oxidizing agent. The resulting sample solution was applied for ICP-AES analysis of titanium, vanadium, chromium, manganese, nickel, copper, and molybdenum. The decomposition condition for the present sample preparation was able to be well optimized for the quantitative analysis of high-carbon iron.