2021 Volume 61 Issue 8 Pages 2264-2273
2021 Volume 61 Issue 8 Pages 2264-2273
It is known that Al added to the Zn coating layer of hot-dip galvanized steel sheets (HDG) diffuses to the surface at room temperature and forms Al-based oxides in air. In order to understand the diffusion behavior of Al in Zn coating layer, this study investigated the effect of the aging temperature on the segregation behavior of Al-based oxides in HDG with and without temper rolling (skinpass rolling) using a material with a Zn coating weight of about 56 g/m2 with an Al content of approximately 0.20 mass%. The specimens were aged at −15, 5, 20, 38, 100 or 200°C in air after production, and the surface and cross sections were observed and analyzed by XRF, SEM-EDX, EBSD and TEM. As a result, up to the aging temperature of 38°C, the amount of Al-based oxides increased linearly to the square root of aging time, suggesting that the formation rate is determined by the diffusion of Al in Zn coating layer in this temperature range. However, this linear relationship did not hold at aging temperatures above 100°C. In addition, in the temper-rolled HDG, the formation rate of Al-based oxides is larger than that without temper rolling up to the aging temperature of 38°C, and then decreased drastically at aging temperatures above 100°C. The segregation behavior of Al-based oxides is discussed in view of the diffusion behavior of Al and the changes in the macrostructure of the Zn coating layer during the aging after production.
Hot-dip galvanized steel sheets (HDG) are widely used as automotive body parts to improve corrosion resistance.1,2) The frictional characteristics of HDG, which affect press formability, are recognized as an important property because automotive components are usually press-formed.3,4,5,6,7) It is well known that Al-based oxides exist on the surface of HDG,8) and the effect of these oxides on the frictional characteristics of HDG has been investigated.9,10,11) It has been reported that Al-based oxides had the effect of reducing the friction coefficient by adhering to the tool surface during sliding.9,10,11) A new high-lubricity HDG was developed by applying this mechanism, by which a thin oxide layer reduces the friction coefficient, and is used practically to stabilize press formability in mass production of automotive body parts.12,13,14)
Previous research on the formation behavior of the Al-based oxides showed that the amount of Al-based oxides increases with increasing aging time after production.15) Generally, a small amount of Al, typically about 0.2 mass%, is added to the molten Zn bath used in hot-dip galvanizing in order to suppress alloying of the Zn coating layer with the substrate steel.16,17,18,19,20) As a result, the Zn coating layer contains a small amount of Al, and it is thought that the Al in the Zn coating layer diffuses from the coating layer to the sheet surface via the grain boundaries of the Zn as aging time increases, and then forms Al-based oxides when exposed to air.21) Since the solubility of Al in solid Zn is known to be quite small,22,23) the Al in the Zn coating layer is supersaturated. This is thought to be one reason why Al diffuses rapidly at room temperature.21)
HDG is usually temper rolled (skinpass rolled) after hot-dip galvanizing to reduce yield elongation. The effect of temper rolling on the formation behavior of Al-based oxides has also been investigated, and it has been reported that temper rolling accelerates the formation of Al-based oxides per unit area because the density of diffusion paths of Al in the Zn coating layer is increased by temper rolling.21) Contact with the temper roll causes dislocations in the Zn coating layer, and then induces recrystallization of the Zn even at room temperature due to the low beginning temperature of recrystallization of Zn alloys.21) As a result, the density of the grain boundaries is increased by contact with the temper roll, although some grains remain unrecrystallized and include diffusion paths such as dislocations.21) Consequently, it is thought that the rate of Al-based oxide formation is accelerated due to an increase in the formation site of Al-based oxides on the surface.21)
As mentioned above, control of Al-based oxides is very important for obtaining stable HDG quality, particularly frictional properties. The segregation mechanism of Al-based oxides on the surface of HDG is explained by diffusion of Al in the Zn coating layer. However the diffusion behavior of Al in the Zn coating layer during the aging have not been clarified yet. In order to understand the diffusion behavior of Al in the Zn coating layer, in this study, we investigated the effect of the aging temperature on the formation behavior of Al-based oxides on the surface of HDG. Furthermore, we discussed the mechanism from the viewpoints of the diffusion behavior of Al in the Zn coating layer with the changes in the macrostructure of the Zn coating layer depending on the aging temperature.
Cold rolled steel sheets with a thickness of 0.7 mm were prepared. The sheets were annealed, galvanized and then cooled. The coating weight and concentration of Al in the Zn coating layer except the Al–Zn intermetallic layer were controlled to approximately 56 g/m2 and 0.2 mass%, respectively. Some galvanized specimens were temper rolled immediately after galvanizing, and some were held without temper rolling. In temper rolling, a rolling force of 0.19 kN/mm and elongation of 0.7% were employed. Rolling was carried out in air at room temperature with lubricant water. After rolling, the lubricant water was dried by hot air with a temperature of 60°C for 5 s. The specimens were degreased with alcohol, and then were aged at −15, 5, 20, 38, 100 or 200°C in air for a prescribed time. Aging time was counted from the time when temper rolling was completed for the temper-rolled HDG or the time when hot-dip galvanizing was completed for the HDG without temper rolling.
2.2. Surface Observation and AnalysisThe intensity of the Al peaks of the HDG specimens was measured with an X-ray fluorescence spectrometer (XRF; ZSX-101E, Rigaku). A calibration curve was prepared in order to calculate the amount of Al from the measured intensity. The calibration curve was made based on the intensity of reference steel sheets on which metallic Al had been vapor deposited, and the amount of Al of the HDG specimens was calculated using the measured peak intensity and the calibration curve. The measured area was a circle with a diameter of 30 mm, and a tube voltage of 45 kV and tube current of 45 mA were employed. Measurements were performed at aging time steps of 24, 72, 120, 192, 360, 696, 1200, 1680 and 3288 h using the same specimens throughout all the measurements.
The surfaces of the HDG specimens aged for 1680 h (70 d) were observed with a field emission scanning electron microscope (FE-SEM; ULTRA Plus, Carl Zeiss), having both secondary electron (SE) and backscattered electron (BSE) detectors. After observation, the surfaces of the HDG specimens were also analyzed with an energy dispersive X-ray spectroscopy (EDX) incorporated in the FE-SEM. The acceleration voltage of 5 kV was used in both the FE-SEM observation and the EDX analysis.
2.3. Cross-sectional Observation and Analysis45° cross-sectional specimens of the HDG aged for 1680 h (70 d) were prepared by a focused ion beam (FIB) instrument (Quanta200 3D, FEI), and the 45° cross-sectional specimens were observed with the above-mentioned FE-SEM and analyzed by EDX. The BSE detector was used in these observations. The observation and analysis were performed with an acceleration voltage of 5 kV.
90° cross-sectional specimens of the HDG aged for 1680 h (70 d) were prepared by an Ar ion milling system (PECSII, GATAN). Electron backscatter diffraction (EBSD) patterns of the Zn crystal grains of the 90° cross-sectional specimens were obtained with a Hikari High Speed EBSD, EDAX incorporated in an FE-SEM (SUPRA 40VP, Carl Zeiss). The EBSD analysis was conducted with a step size of 0.05 μm and acceleration voltage of 20 kV. The obtained data were analyzed with the dedicated processing software (OIM Matrix, EDAX).
90° cross-sectional specimens of the temper-rolled HDG aged for 1680 h (70 d) were prepared by above-mentioned FIB instrument, and the 90° cross-sectional specimens were observed with the transmission electron microscope (TEM) and analyzed by EDX. The scanning transmission microscopy (STEM) mode was used in these observations. The observations and analysis were carried out with an acceleration voltage of 200 kV.
Figure 1 shows the amount of Al on the surface of the HDG without temper rolling as a function of the square root of aging time. From previous reports,9,10,11,21) it can be said that the amount of Al measured by XRF before aging is a background value, and the increased amount resulting from aging is the Al content of the Al-based oxides on the surface of the HDG. The amount of Al-based oxides increased as the aging time increased at all aging temperatures used in this study. The amount of Al-based oxides also increased with aging temperature, suggesting that the diffusion rate of Al in the Zn coating layer affected the formation rate of the Al-based oxides. The formation rate of the Al-based oxides was linear to the square root of aging time up to the aging temperature of 38°C. Therefore, the formation rate of the Al-based oxides is determined by the diffusion rate of Al from the Zn coating layer to the surface of the HDG. However, above 100°C, the amount of Al-based oxides increased nonlinearly to the square root of aging time, which implies that the rate-determining process changed in this temperature region.

Amount of Al on surface of HDG without temper rolling measured by XRF as function of square root of aging time.
Figure 2 shows the amount of Al on the surface of the temper-rolled HDG as function of the square root of aging time. As in Fig. 1, the amount of Al measured by XRF before aging is a background value. Therefore, the increase of the amount of Al due to aging is the Al content of the Al-based oxides on the surface of the HDG. The amount of Al-based oxides increased as the aging time increased at all aging temperatures. This behavior was the same as in the HDG without temper rolling. The formation rate of Al-based oxides increased as the aging temperature increased up to 38°C, however, at aging temperatures above 100°C, the formation rate of the temper-rolled HDG decreased drastically as the aging temperature increased. The amount of Al-based oxides increased linearly to the square root of aging time up to 38°C, suggesting that the formation rate of the Al-based oxides is determined by the diffusion rate of Al from the Zn coating layer in this temperature region. From the comparison between Figs. 1 and 2, the formation rate of Al-based oxides of the temper-rolled HDG was larger than that of the HDG without temper rolling. This can be explained by the difference of the formation site of the Al-based oxides.21) Our previous research showed that Al-based oxides segregate in the temper-rolled area of the HDG surface because the crystal grains of the Zn coating layer are refined by recrystallization owing to deformation by temper rolling, and some grains include dislocations which provide diffusion paths for Al.21) However, above 100°C, the formation rate of the Al-based oxides was nonlinear to the square root of aging time, suggesting that the rate-determining process changed in this temperature region. It may also be noted that the amount of Al-based oxides in the temper-rolled HDG aged at 200°C converged with that of the HDG without temper rolling aged at 200°C as the aging time increased.

Amount of Al on surface of temper-rolled HDG measured by XRF as function of square root of aging time.
In order to identify the initial surface of the HDG specimens before aging, Fig. 3 shows the results of SEM observation of the surfaces of both the HDG without temper rolling and the temper-rolled HDG. Table 1 shows the results of an elemental composition analysis of the center of squares (1) and (2) in Fig. 3 by EDX. The HDG without temper rolling exhibits a typical solidification microstructure of Zn alloys, and Al-based oxides were not observed on the grain boundaries, as shown in Figs. 3(a), 3(b) and 3(c). In the temper-rolled HDG, both a partially uneven surface caused by temper rolling and the solidification microstructure of Zn alloys were observed on the surface. Al-based oxides were not detected clearly on the uneven surface or grain boundaries, as shown in Figs. 3(d), 3(e) and 3(f). These results suggest that the initial amounts of Al observed in Figs. 1 and 2 were due to solid-solute Al in the Zn coating layer, namely background Al.
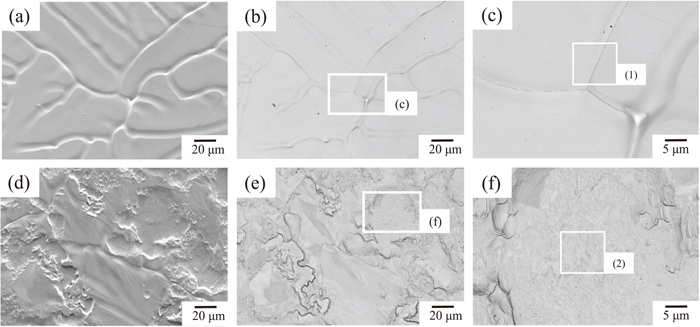
Result of SEM surface observation of HDG without temper rolling and temper-rolled HDG before aging. (a) SE image of HDG without temper rolling, (b) BSE image of (a), (c) high magnification image of white square in (b); (d) SE image of temper-rolled HDG, (e) BSE image of (d), and (f) high magnification image of white square in (e).
| No. | Analytical results (at%) | ||
|---|---|---|---|
| O | Al | Zn | |
| 1 | 4.1 | 2.7 | 93.2 |
| 2 | 4.3 | 2.3 | 93.4 |
Figure 4 shows the BSE images of the surface of the HDG without temper rolling after aging at 5, 20, 38 or 200°C for 1680 h, and Table 2 lists the EDX analytical results for the center of squares (1) to (8) in Fig. 4. Two areas with different contrasts were observed in the BSE images in Fig. 4. One is an area of bright contrast, which can be identified as the metallic Zn alloy from the EDX results, as shown in squares (2), (4), (6) and (8) in Fig. 4. The other is an area of dark contrast, which can be identified as Al-based oxides, as shown in squares (1), (3), (5) and (7) in Fig. 4. The locations of the Al-based oxides coincide with the grain boundaries of the Zn. This phenomenon agrees with our previous report,21) which investigated the segregation behavior of Al-based oxides at an aging temperature of 20°C. Al-based oxides formed on the grain boundary at all aging temperatures, meaning that the formation site is independent of the aging temperature. The thickness of the Al-based oxides increased with the aging temperature up to 38°C, corresponding to the increase in the amount of Al measured by XRF (Fig. 1). Al-based oxides also formed on the grain boundaries at 200°C, but were thinner at that temperature than at 38°C.

BSE images of surface of HDG without temper rolling aged at (a) 5°C, (b) 20°C, (c) 38°C and (d) 200°C for 1680 h.
| No. | Analytical results (at%) | ||
|---|---|---|---|
| O | Al | Zn | |
| 1 | 20.2 | 6.1 | 73.7 |
| 2 | 4.3 | 3.0 | 92.7 |
| 3 | 25.3 | 10.4 | 64.3 |
| 4 | 4.4 | 1.4 | 94.2 |
| 5 | 37.0 | 19.2 | 43.8 |
| 6 | 4.1 | 1.8 | 94.1 |
| 7 | 17.7 | 10.5 | 71.8 |
| 8 | 4.5 | 1.8 | 93.7 |
Figure 5 shows the BSE images of the cross sections of the HDG without temper rolling after aging at 20, 38 or 200°C for 1680 h, and Table 3 summarizes the EDX analytical results of the center of squares (1) to (8) in Fig. 5. Two areas with different contrasts were also observed in the cross sections in Fig. 5. One is an area of bright contrast, which can be identified as the metallic Zn alloy from the EDX results (square (8) in Fig. 5.), and the other is the area of dark contrast. The area of dark contrast can be divided into Al-based oxides and metallic Al. The area of dark contrast on the surface can be identified as Al-based oxides because both Al and O were detected in the EDX analysis. Al-based oxides existed on the surface of the grain boundaries, as shown in squares (2), (5) and (7) in Fig. 5. The appearance of the Al-based oxides of the HDG aged at 20°C and 38°C was similar, but the size was larger at 38°C. At 200°C, the Al-based oxides appeared thinner and deeper than at 20°C and 38°C. Another area of dark contrast in the Zn coating layer can be identified as metallic Al because O was not detected in the EDX analysis, as shown in squares (1), (3), (4) and (6) in Fig. 5. Our previous report21) clarified the fact that α-Al precipitates in the Zn coating layer after aging at 20°C because Al is supersaturated immediately after galvanizing due to the low solubility of Al in Zn, which is considerably less than 0.2 mass% at 20°C. Thus, the observed metallic Al would be α-Al that precipitated after aging due to the supersaturation of Al in the Zn coating layer. Although α-Al was precipitated in the Zn coating layers aged at 20°C and 38°C, α-Al precipitation was not observed at 200°C.
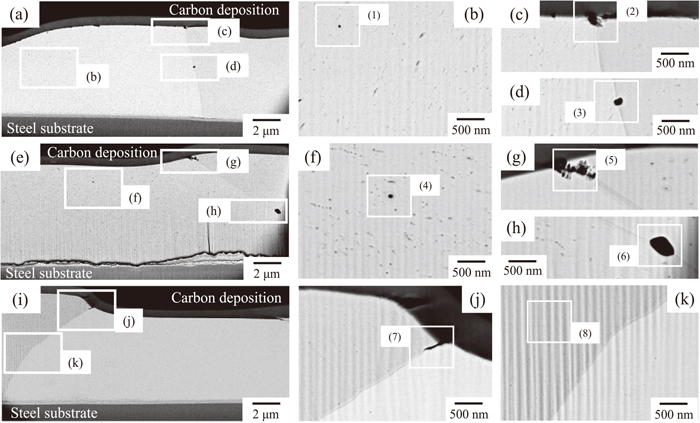
45° cross-sectional BSE images of HDG without temper rolling aged at (a) 20°C, (e) 38°C and (i) 200°C for 1680 h, (b, c, d) results of high magnification observation of (a); (f, g, h) results of high magnification observation of (e), and (j, k) results of high magnification observation of (i).
| No. | Analytical results (at%) | ||
|---|---|---|---|
| O | Al | Zn | |
| 1 | 3.6 | 18.0 | 78.4 |
| 2 | 26.9 | 15.9 | 57.2 |
| 3 | 1.2 | 49.7 | 49.1 |
| 4 | 0.8 | 31.7 | 67.5 |
| 5 | 33.1 | 20.8 | 46.1 |
| 6 | 1.1 | 85.6 | 13.3 |
| 7 | 29.5 | 13.7 | 56.8 |
| 8 | 2.3 | 0.5 | 97.2 |
Figure 6 shows the BSE images of the surface of the temper-rolled HDG after aging at 5, 20, 38 or 200°C for 1680 h, and Table 4 shows the EDX analytical results for the center of squares (1) to (11) in Fig. 6. Here, the area in contact with the temper roll was the main focus of observation. Bright-contrast metallic Zn (squares (3), (6), (9) and (11) in Fig. 6) and dark-contrast Al-based oxides were observed on the surface of the temper-rolled HDG, as shown in Fig. 6. In our previous report,21) we found that Al-based oxides segregated on grain boundaries formed by recrystallization due to contact with the temper roll and on grains that remained unrecrystallized, which contained dislocations. For example, Al-based oxides are observed on the grain boundaries formed by recrystallization, as shown in squares (2), (5) and (8) in Fig. 6, and Al-based oxides are also observed on unrecrystallized grains containing dislocations, as shown in squares (1), (4) and (7) in Fig. 6. The behavior up to 38°C agrees with the behavior described in the previous report,21) which investigated the segregation of Al-based oxides at 20°C. Al-bases oxides were observed on both grain boundaries and unrecrystallized grains up to the aging temperature of 38°C. However, Al-based oxides were not observed on unrecrystallized grains in the material aged at 200°C, but were observed on the grain boundaries at this temperature, as shown in square (10) in Fig. 6.

BSE images of surface of temper-rolled HDG aged at (a) 5°C, (b) 20°C, (c) 38°C and (d) 200°C for 1680 h.
| No. | Analytical results (at%) | ||
|---|---|---|---|
| O | Al | Zn | |
| 1 | 31.1 | 11.9 | 57.0 |
| 2 | 24.8 | 10.5 | 64.7 |
| 3 | 5.4 | 2.6 | 92.0 |
| 4 | 35.9 | 15.5 | 48.6 |
| 5 | 27.5 | 11.7 | 60.8 |
| 6 | 5.3 | 1.9 | 92.8 |
| 7 | 37.7 | 17.6 | 44.7 |
| 8 | 35.8 | 16.5 | 47.7 |
| 9 | 4.1 | 2.2 | 93.7 |
| 10 | 19.1 | 7.3 | 73.6 |
| 11 | 6.7 | 3.5 | 89.8 |
The BSE images of the cross sections of the temper-rolled HDG aged at 20, 38 or 200°C for 1680 h are shown in Fig. 7. The EDX analytical results are summarized in Table 5 for the center of squares (1) to (8) in Fig. 7. The area in contact with the temper roll was the main focus of observation. Bright-contrast metallic Zn (squares (5) and (8) in Fig. 7), dark-contrast Al-based oxides near the surface (squares (1), (4) and (7) in Fig. 7), and dark-contrast α-Al in the Zn coating layer (squares (2), (3) and (6) in Fig. 7) were observed in the cross sections. At aging temperatures of 20°C and 38°C, the grain size of the temper-rolled Zn coating layer was finer than that without temper rolling (Fig. 5) due to the recrystallization induced by temper rolling.21) Al-based oxides existed on the surface of the grain boundaries at all aging temperatures. However, α-Al was not observed in the Zn coating layer after aging at 200°C, although it was observed in the specimens aged at 20°C and 38°C. This result corresponded with that of the HDG without temper rolling (Fig. 5). After aging at 200°C, the grain size of the Zn coating layer was larger than after aging at 20°C and 38°C, and was close to the grain size without temper rolling (Fig. 5).

45° cross-sectional BSE images of temper-rolled HDG aged at (a) 20°C, (d) 38°C and (g) 200°C for 1680 h, (b, c) results of high magnification observation of (a), (e, f) results of high magnification observation of (d), and (h) results of high magnification observation of (g).
| No. | Analytical results (at%) | ||
|---|---|---|---|
| O | Al | Zn | |
| 1 | 21.1 | 11.9 | 67.0 |
| 2 | 1.0 | 16.5 | 82.5 |
| 3 | 2.1 | 43.8 | 54.1 |
| 4 | 29.3 | 18.5 | 52.2 |
| 5 | 3.5 | 1.1 | 95.4 |
| 6 | 0.6 | 59.7 | 39.7 |
| 7 | 16.0 | 13.0 | 71.0 |
| 8 | 2.4 | 0.3 | 97.3 |
In order to understand why α-Al was not observed in the Zn coating layer of the specimens aged at 200°C in either the HDG without temper rolling or the temper-rolled HDG (Figs. 5 and 7), a Zn–Al phase diagram was calculated, as shown in Fig. 8. Thermo-Calc software24) and thermodynamic parameters assessed by one of the authors25) were used in the calculation. As shown in the figure, the solubility of Al in Zn increases as the temperature increases. Although 0.2 mass% of Al is supersaturated in the Zn coating layer at temperatures below approximately 160°C, it is obvious that 0.2 mass% of Al is soluble in Zn at 200°C. Therefore, α-Al was not observed in the cross-sectional observation of either the HDG without temper rolling or the temper-rolled HDG aged at 200°C (Figs. 5 and 7).

Calculated Zn–Al phase diagram.
To clarify the recrystallization behavior of the Zn coating layer, EBSD patterns of the cross sections of HDG specimens after aging at 20, 38 or 200°C for 1680 h were obtained. The inverse pole figure (IPF) and grain reference oriented deviation (GROD) maps of the HDG without temper rolling and the temper-rolled HDG are shown in Figs. 9 and 10, respectively. In the GROD technique, the gap of the orientation in a pixel from the average orientation of a grain is shown as a GROD value. Although no major difference depending on the aging temperature was observed in the HDG without temper rolling, as shown in Fig. 9, a significant change in the microstructure of the temper-rolled HDG can be seen in Fig. 10. The authors previously reported that the grains of the Zn coating layer were refined by contact with the temper roll at room temperature21) due to the low beginning temperature of Zn recrystallization.26) Some unrecrystallized grains also remained because recrystallization was not completed during temper rolling, and these unrecrystallized grains have a higher GROD value because the grain orientation is ununiform due to dislocations in the grains.21) Actually, the grain size of the temper-rolled HDG aged at temperatures lower than 38°C is finer than that of the HDG without temper rolling, and some grains had high GROD values owing to dislocations in the grains, as shown in Figs. 10(a), 10(b), 10(d) and 10(e). However, at 200°C, the grain size of the HDG is larger than that of the HDG aged at 20°C and 38°C and is close to that of HDG without temper rolling (Fig. 9), and grains with high GROD values was not observed, as shown in Figs. 10(c) and 10(f).

90° cross-sectional IPF maps of Zn coating layer of HDG without temper rolling aged at (a) 20°C, (b) 38°C and (c) 200°C for 1680 h and (d, e, f) GROD maps of (a), (b) and (c).

90° cross-sectional IPF maps of Zn coating layer of temper-rolled HDG aged at (a) 20°C, (b) 38°C and (c) 200°C for 1680 h and (d, e, f) GROD maps of (a), (b) and (c).
The recrystallization temperature of the Zn alloy was investigated by Kyotani,17) who reported that the beginning temperature of recrystallization is between 10°C and 50°C and the finishing temperature of the recrystallization is between 80°C and 100°C, depending on the purity of the Zn alloy.26) This means that recrystallization of the Zn coating layer is not completed during temper rolling, and proceeds during aging at temperatures above 100°C. As a result, unrecrystallized grains were not observed in the cross sections of the temper-rolled HDG aged at 200°C, and the grains grew to a larger size than in the material aged at less than 38°C. These changes in the microstructure can be expected to affect the segregation behavior of the Al-based oxides because the density of the diffusion paths is decreased by recrystallization and grain growth.
The formation rate of Al-based oxides of the HDG without temper rolling increased as the aging temperature increased up to 200°C, as shown in Fig. 1. This can be explained by the fact that the microstructure did not show any significant difference depending on the aging temperature. However, in the temper-rolled HDG, the formation rate of Al-based oxides decreased as the aging temperature increased above 100°C, even though it had increased with the aging temperature in the temperature range up to 38°C, as shown in Fig. 2. This change can be attributed to the decreasing density of Al diffusion paths in Zn coating layer due to the completion of recrystallization and grain growth in the Zn coating layer, as explained above.
4.3. Oxidation Behavior of Zn during Aging at Increased TemperatureAs shown in Figs. 1 and 2, the formation rate of the Al-based oxides was nonlinear to the square root of aging time above 100°C of aging temperature, whereas the amount of the Al-based oxides increases linearly to the square root of aging time up to 38°C for both HDG without temper rolling and temper-rolled HDG. In order to understand this behavior, the compositions of the Al-based oxides were investigated in detail. Figure 11 shows the TEM bright field images of the cross sections of the temper-rolled HDG aged at 20 or 200°C for 1680 h. The results of EDX mapping are also shown in the figure. At the aging temperatures of 20°C, the distributions of Al and O were completely the same, suggesting that the Al-based oxides consist of Al and O, mainly. However, at the aging temperatures of 200°C, the detected area of O was wider than that of Al. This suggests that the oxides contains Zn as well as Al at the aging temperatures of 200°C. Generally, it is known that Al is easier oxidizable element than Zn. In the case for lower aging temperature such as below 38°C, Al is oxidized selectively and then the Al-based oxides formed on the surface on the HDG, meaning that the oxidation of Zn is negligible. However, in the case of higher aging temperature such as above 100°C, Zn is also oxidized because the amount of Zn on the surface of the HDG is much larger than that of Al and Zn tend to be oxidized with ease at the increased temperature. This oxidation behavior of Zn probably affected the segregation behavior of the Al-based oxides. Below 38°C, O is simply consumed by oxidation of Al and the oxidation of Zn is negligible. As a result, the amount of Al-based oxides increases linearly to the square root of aging time at all aging times used in this study, as shown in Figs. 1 and 2, because the formation rate of Al-based oxides is determined by the diffusion rate of Al in Zn coating layer. However, above 100°C, O is consumed by the oxidation of both Zn and Al. In addition the diffusion behavior of O is also changed depending on the compositions of the formed oxides. As a result, the formation rate of Al-based oxides are no longer determined by the diffusion rate of Al in Zn coating layer, leading to the nonlinear increase in the Al-based oxides against the square root of aging time, as shown in Figs. 1 and 2.

90° cross-sectional TEM bright field images of Al-based oxides of temper-rolled HDG aged at (a) 20°C and (b) 200°C for 1680 h. EDX intensity maps of Al, O and Zn are shown right side of the TEM images.
To elucidate the rate-determining steps of the formation rate of the Al-based oxides, the activation energy of Al diffusion in Zn coating layer was assumed. Clarification of the rate-determining steps above the aging temperature of 100°C is difficult because the density of the diffusion paths is not constant during aging and the oxidation of Zn is not negligible, as discussed in Sections 4.2 and 4.3. Thus, the rate-determining step up to 38°C was assumed. In order to calculate activation energy, the diffusion coefficient must be determined. Therefore, the apparent diffusion coefficient was assumed by assuming the diffusion coefficient from the amount of oxides formed on the metallic surface uniformly, as proposed by Sasabe.27,28,29) Here, the calculated diffusion coefficient is simply an apparent diffusion coefficient which is different from the actual diffusion coefficient because the Al-based oxides are localized on the HDG surface.
The relationship between the amount of Al in the Al-based oxides per unit area and the diffusion coefficient can be expressed as Eq. (1).
| (1) |
| (2) |
| (3) |

Arrhenius plots of calculated apparent diffusion coefficients and aging temperature.
From the constant of proportionality in Fig. 12 and Eq. (4),30) the activation energy of Al diffusion in Zn coating layer can be calculated as follows.
| (4) |
The models of diffusion behavior of Al and Al-based oxide segregation depending on the aging temperature are summarized in Fig. 13 as schematic images. Up to 38°C, the Al-based oxides segregate on the surface of the HDG by the same mechanism as described in our previous report.21) In the HDG without temper rolling, the supersaturated Al in the Zn coating layer diffuses from the bulk to the grain boundaries, after which some Al precipitates as α-Al and other Al diffuses through the grain boundaries to the surface of the HDG to form Al-based oxides. In the temper-rolled HDG, the supersaturated Al in the Zn coating layer diffuses from the bulk to original grain boundaries, grain boundaries of recrystallized grains and dislocations of the unrecrystallized grains. Some of this Al precipitates as α-Al and other Al diffuses to the surface of the HDG via grain boundaries and dislocations, and then forms Al-based oxides. The formation rate of the Al-based oxides per unit area of the temper-rolled HDG appears to be larger than that of the HDG without temper rolling because the formation site of the Al-based oxides in the temper-rolled HDG is larger than that of the HDG without temper rolling due to the larger density of diffusion paths in the temper-rolled HDG. As described in the previous section, the calculated activation energies of Al diffusion in Zn coating layer of the HDG without temper rolling and the temper-rolled HDG at aging temperatures of less than 38°C are almost the same. This suggests that the diffusion behavior of Al in Zn coating layer is essentially the same between the HDG without temper rolling and temper-rolled HDG although the density of the diffusion paths is different. In addition, the calculated activation energies are similar to the activation energies of body diffusion of Cd, Ga, Hg, In, Sn in Zn, suggesting that the formation rate of the Al-based oxides is determined by body diffusion of Al in Zn coating layer. The rate-determining step of body diffusion of Al in Zn coating layer at aging temperatures of less than 38°C could be explained by the fact that grain boundary diffusion is faster than body diffusion.

Schematic images of models of diffusion of Al and microstructure of Zn coating layer depending on aging temperature.
Above 100°C, the diffusion rate of Al in Zn coating layer could increase as the aging temperature increased. However, the oxidation of Zn in Zn coating layer is not negligible as the aging temperature is increasing. This would affect the oxidation behavior of Al in Zn coating layer, and the rate-determining step would change depending on the aging time because O is consumed by the oxidation of Zn as well as Al. As a result, the Al-based oxide formation rate is no longer proportional to the square root of aging time, as shown in Figs. 1 and 2. Moreover, in the temper-rolled HDG, unrecrystallized grains are recrystallized by aging at temperatures above 100°C, and the recrystallized fine grains grow to a large size. This means the microstructure also changes depending on the aging temperature, and this change reduces the density of Al diffusion paths to the surface. Therefore, in the temper-rolled HDG, the formation rate of the Al-based oxides decreases drastically at aging temperatures over 100°C, as shown in Fig. 2.
In order to understand the diffusion behavior of Al in Zn coating layer, the effect of the aging temperature on the segregation of Al-based oxides on the surface of Zn-0.2 mass% Al hot-dip galvanized steel sheets (HDG) was investigated.
(1) For the HDG without temper rolling, the formation rate of the Al-based oxides increased as the aging temperature increased. Up to 38°C, the Al-based oxides increased linearly to the square root of aging time. This suggests that the formation rate of the Al-based oxides is determined by the diffusion of Al in Zn. However, above 100°C, the increase in Al-based oxides was not proportional to the square root of aging time because the oxidation of Zn would affect the oxidation behavior of Al.
(2) As in the case of HDG without temper rolling, the formation rate of Al-based oxides in the temper-rolled HDG increased as the aging temperature increased up to 38°C, and the formation rate was proportional to the square root of the aging time, suggesting that the formation rate of Al-based oxides is determined by the diffusion of Al in Zn in this temperature range. However, above 100°C, the formation rate of Al-based oxides decreased drastically as the aging temperature increased. This sharp decrease is explained by the fact that the unrecrystallized grains of Zn were recrystallized completely by aging at above 100°C, and the recrystallized Zn grains grew to a large size, reducing the density of the diffusion paths for Al in the Zn coating layer as the aging temperature increased. Above 100°C, the formation rate of the Al-based oxides is not linear to the square root of aging time, as in the case of the HDG without temper rolling. As in the HDG without temper rolling, this is attributed to the oxidation of Zn at higher temperatures.
(3) The activation energy of Al diffusion in Zn coating layer calculated from the results of specimens aged at temperatures of less than 38°C was 72.3 kJ/mol for the HDG without temper rolling and 69.6 kJ/mol for the temper-rolled HDG. This suggests that the diffusion behavior of Al in Zn coating layer is essentially the same between the HDG without temper rolling and temper-rolled HDG although the density of the diffusion paths is different because these calculated values are almost the same. These calculated values are also close to the activation energies of the body diffusion of other elements such as Cd, Ga, Hg, In, Sn in Zn. This suggests that the formation rate of Al-based oxides is determined by the body diffusion of Al in Zn from the bulk to the grain boundary for both the HDG without temper rolling and temper-rolled HDG.