2022 Volume 62 Issue 1 Pages 1-11
2022 Volume 62 Issue 1 Pages 1-11
In order to comprehensively utilize copper smelting slag, the effect of strengthening measures on the reduction rate of the copper smelting slag, reduction kinetics, magnetic separation of reduced pellet and volatilization of residual valuable constituents were investigated in the present study. Milling for mechanical activation was the most efficient method to improve the reduction rate of copper smelting slag compared to addition of Na2CO3 catalyst and high reactivity reducing agent. The metallization degree of reduced pellet increased from 54.5% to 75.5% when the slag-coal mixture was milled for 30 s and reduced at 1100°C for 30 min. The apparent activation energy for the reduction of milled pellet increased from 96.1 kJ/mol to 153.5 kJ/mol. The iron concentrate magnetically separated from the milled pellet reduced at 1200°C had the best quality. The removal rates of typical elements during direct reduction-magnetic separation decreased in the sequence of Zn>K>Na>Cu. Secondary-dust captured from the flue gas contained 70.17 mass% Zn and 11.99 mass% Pb, which could meet the requirements of I grade zinc ore. The Zn in the dust existed in the form of ZnO. The productivity of the dust was around 1.49%. The application of mechanical milling in the reduction of copper smelting slag/coal composite pellet can improve the reduction efficiency of iron oxide and the quality of Zn-rich secondary dust. This work can help to enhance the utilization of copper smelting slag in a more efficient and sustainable method.
Copper is one of the most important nonferrous metals consumed in our daily life. It is estimated that 80% of the global metallic copper is produced via pyrometallurgical technologies.1) Furthermore, the relative data value is 97% in China.2) The oxides in the copper concentrate and fuel ash will melt together forming the so-called copper smelting slag, which contains SiO2, FeO, CaO, Al2O3, MgO as well as some valuable elements, such as Zn, Cu, Co and Ni.2) The productivity of copper smelting slag is 2.2 times as much as the metallic copper.3) In each year, approximately 24.6 million tons of slags are generated from world copper production, about 7 million tons of which are produced in China.3,4) Dumping and disposal of these slags cause the wastage of valuable metals, such as Fe, Zn, Cu, Co and Ni, and lead to the environmental problems as well as the space shortage.2,3) Therefore, the comprehensive utilization of the copper smelting slag is highly significant for the sustainable development of copper manufacturing industry.
Lots of attempts have been made by researchers and copper producing enterprises all over the world to develop efficient methods for the utilization of copper smelting slag. Generally, there are five main directions for the utilization of copper smelting slag, such as pyrometallurgical methods,5,6,7,8,9) flotation,10,11) hydrometallurgical leaching,3,12,13,14,15) combination of pyro-hydrometallurgical methods3,16) and direct utilization.17) The pyrometallurgical treatment of copper smelting slag is mainly performed through direct reduction of oxide components, such as Cu2O and PbO, by coal, coke, gaseous reducing agents, waste oil or biomass in an electric furnace for copper recovery. The obtained product Cu–Fe–Pb alloy is then conveyed to the converter process where the lead and iron are oxidized. The copper can also be recycled in the form of Cu2S by adding FeS during reduction. The metallic copper and copper sulfide can be enriched by flotation technology after slow cooling and grinding of the copper smelting slag. The hydrometallurgical leaching method can extract most of the valuable nonferrous metals from various grades of raw or pretreated copper smelting slag by H2SO4, HCl, NH4Cl, Cl2, etc. Furthermore, copper smelting slag can be directly used as raw materials for clinker, cement replacement, coarse and fine aggregates in the production of cement, mortar and concrete. It can help save substantial amount of energy and provide economic benefits for related industries. The pyrometallurgical and flotation methods have been widely used in the industrial production for processing of primary copper smelting slag with relatively higher content of copper, lead and zinc. However, all the above methods cannot recycle the iron element, which accounts for the largest mass fraction in the slag and is discarded after copper recovery. It is well known that China has the largest iron and steel output in the world. The iron ore used in ironmaking relies heavily on imported resource for Chinese steel industry. Therefore, there is the potential to use the copper smelting slag as raw material resource for ironmaking.
After copper enrichment through the pyrometallurgical or flotation methods, the iron element will still aggregate in the tailings. The Fe-rich tailings should be further utilized for the purpose of iron recycle and environmental protection. In recent years, increasing attention has been paid to the carbon composite iron ore agglomerates direct reduction technology by many strategists and researchers in ironmaking and waste management industry.18,19) The iron and zinc enrichment technology has been proposed,20,21) which is through coal-based direct reduction in a rotary hearth furnace (RHF) and following magnetic separation method using the Fe-rich tailings of copper smelting slag as raw material. Basically, the higher reduction degree is of great significance for the following iron enrichment stage. To the best of our knowledge, the fundamental study on the improvement of coal-based direct reduction of copper smelting slag is little in the literature.
The main purpose of this study is to examine the effect of some strengthening measures on the reduction of the Fe-rich copper smelting slag. The reduction kinetics analysis, magnetic separation of reduced pellet and volatilization of residual valuable constituents, such as Zn, Pb, K and Na, have also been conducted.
The chemical composition of copper smelting slag is given in Table 1, which illustrates that it contains high content of T.Fe, Zn, Cu and SiO2 as well as S. The mineralogical analysis of copper smelting slag was investigated by XRD. The result in Fig. 1 indicates that the main crystalline phases are fayalite (Fe2SiO4) and magnetite (Fe3O4). The particle size distribution of the slag analyzed by a laser particle analyzer is shown in Fig. 2. The particle size of the slag is very fine, and about 90% is less than 10.6 μm. Its volume-average particle diameter is 3.6 μm, and the specific surface area is 2.9 m2/cm3. The microstructure and chemical content of powdery copper smelting slag investigated by SEM-EDS are illustrated in Fig. 3. It can be seen that there are three main phases in the slag based on the SEM images. The grey white phase (No. 1) contains Fe and O in large amount and Zn in small amount, which indicates this phase is magnetite. The grey phase (No. 2) contains Fe, Si and O in large amount and Zn in smaller amount, which indicates this phase is fayalite. A dark grey phase (No. 3) also exists in the slag and its chemical composition is more complicated. This dark grey phase could not be analyzed by XRD, which indicates that it might be in glassy state.
| T.Fe | FeO | Fe2O3 | MgO | SiO2 | Al2O3 | CaO | ZnO | K2O | Na2O | Pb | Cu | P | S |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 40.40 | 40.97 | 12.19 | 0.84 | 32.13 | 4.03 | 2.39 | 2.46 | 0.78 | 0.15 | 0.49 | 0.28 | 0.040 | 0.29 |

XRD pattern of copper smelting slag.

Particle size distribution of copper smelting slag sample. (Online version in color.)

SEM and EDS analysis of copper smelting slag. (Online version in color.)
Pulverized anthracite coal and wood char coal were used as the reducing agent in the experiment. The chemical composition of the reducing agent is listed in Table 2. The fineness of the reducing agents is 100% passing 0.5 mm screen. The reactivity of the pulverized coal was assessed by chemical reaction ability between pulverized coal and carbon dioxide (60 mL/min) by thermogravimetric method at a heating rate of 10°C/min. As shown in Fig. 4, the reactivity of char coal is much better than that of anthracite coal. The char coal is almost completely reacted at about 960°C, whereas the corresponding temperature for anthracite coal is about 1200°C.
| Proximate analysis | Ash analysis | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| CFd | Vd | Ad | S | SiO2 | Al2O3 | Fe2O3 | CaO | MgO | |
| Anthracite coal | 81.40 | 6.40 | 11.10 | 0.34 | 46.10 | 32.16 | 9.51 | 4.26 | 0.65 |
| Char coal | 58.80 | 37.53 | 3.66 | 0.05 | 2.58 | 0.77 | 1.56 | 61.86 | 8.60 |
FCd: Fixed Carbon (dry basis), Vd: Volatile Matter (dry basis), Ad: Ash (dry basis).

Reactiveness thermogravimetric curve of the pulverized reducing agent. (Online version in color.)
Sodium carbonate (Na2CO3) of analytical reagent (AR) grade with the purity of 99.8% was used as a catalyst in the study to improve the reduction rate of iron oxide in the copper smelting slag. The particle size of the sodium carbonate ranges from 0.1 mm to 1.0 mm.
2.2. Experimental Procedure 2.2.1. Reduction ExperimentThe copper smelting slag and pulverized reducing agents were completely mixed together with a mole ratio of C/O ([fixed carbon mol (C)]/[reducible oxygen mol (O) (i.e. oxygen contained in iron oxide)]) equaling to 1.0. In order to investigate the effect of additive on the reduction rate, a certain amount of Na2CO3 was added into the mixture of copper smelting slag and anthracite coal, and the ratios were 0.5 mass%, 1.0 mass% and 1.5 mass% of the mixture, respectively. In order to investigate the effect of milling for mechanical activation on the reduction rate, the mixture of copper smelting slag and anthracite coal with the weight of 20.0 g was milled in a vibrating ball mill for 30 s, 60 s and 90 s, respectively. The moisture of the mixture for pelletizing was controlled as 7.0 mass%. The pelletizing process was performed through a manual ball press under the pressure of 20 MPa. The weight of the green pellet was about 10.0 g. The green pellet presented column shape of 20 mm in diameter and 22 mm in height. The pellet was dried at 100°C for 12 h before the reduction experiment. The isothermal reduction experiment was carried out in a thermogravimetric system with a shaft MoSi2 resistance furnace, with a high-purity N2 flow of 5 L/min as the purge gas and one pellet at each run. The pellet was put into a corundum crucible and the crucible was suspended by Fe–Cr–Al–Mo wire attached to an electronic balance and heated at different temperatures. The change of the weight of the sample with time was collected by computer. The total heating time was 30 mins.
2.2.2. Magnetic Separation ExperimentAfter the reduction experiment finished at each run, the crucible was taken out of the furnace tube and cooled down to ambient temperature under the protection of nitrogen. Some of the reduced pellets were ground to 100% passing the 74.0 μm screen for the magnetic separation to reveal the effect of strengthening factors on the recovery behavior of iron from the copper smelting slag. Enough pellets under specific experimental parameters would be prepared by repeated reduction before the magnetic separation experiment. Around 15.0 g powdery sample was mixed with a certain amount of water to form slurry. The slurry was then processed in a XCGS-73 Davies Magnetic Tube to obtain the high T.Fe content concentrate from reduced pellet. The value of magnetic field intensity in the magnetic separation was set to 100 kA/m. The magnetic separation was performed in one step scheme.
The experiment design and main experimental apparatus used in the experiment are schematically illustrated in Fig. 5.

Experiment design and schematic diagram of experimental apparatus. (Online version in color.)
The iron oxides in the copper smelting slag exist mainly in fayalite and magnetite, and the amount of fayalite is much more than of magnetite. Fayalite and magnetite are reduced directly and indirectly.
The indirect reductions:
| (1) |
| (2) |
| (3) |
The direct reductions:
| (4) |
| (5) |
| (6) |
If the pellet is heated to the temperature that the solid carbon can react with CO2 in a relatively fast rate, the indirect reduction reactions will combine with the Boudouard reaction (i.e. 1/2C+1/2CO2=CO) and the reduction of iron oxide by solid carbon will proceed through the gaseous intermediates CO and CO2 in the so-called direct reduction mode. The thermodynamic data for the indirect and direct reductions is listed in Fig. 6.22) The Fe3O4 and FeO can be easily reduced by CO and C. Increasing reducing temperature is obviously beneficial for the direct reduction and Boudouard reaction. In the standard state, the Fe2SiO4 cannot be reduced by CO at all. However, the Fe2SiO4 can be theoretically reduced by solid carbon at the temperature higher than 769°C. It indicates that the reducibility of fayalite is much worse than simple iron oxides, such as Fe3O4 and FeO.

Thermodynamic data for the indirect and direct reduction reactions. (Online version in color.)
The reduction degree of iron oxide is of great significance in the coal-based direct reduction and magnetic separation method for the utilization of copper smelting slag. Hence, the effect of reduction temperature (1000°C, 1050°C, 1100°C, 1150°C, 1200°C) and reduction strengthening measures, such as adding Na2CO3 catalyst (0 mass%, 0.5 mass%, 1.0 mass%, 1.5 mass%), use of high reactivity reducing agent (0%, 20%, 40%, 100%) and milling for mechanical activation (0 s, 30 s, 60 s, 90 s), on the reduction of copper smelting slag/coal composite pellet was investigated. Lots of studies have shown that the improvement of iron ore reduction rate increases in the sequence of graphite<coke<coal<char coal with the increment of the reactivity of reducing agent.23,24) It has been a long history for the metallurgical researchers to investigate the effect of catalyst on the rate of high temperature metallurgical reactions.25,26,27,28) Most of the researches focus on the performance of alkali metals (Li2CO3, K2CO3, Na2CO3, Rb2CO3, etc.) and alkaline-earth metals (CaCO3, SrCO3, etc.). Mechanical milling (i.e. mechanical activation) is also an effective method to accelerate the reaction rate and reduce the reaction starting temperature of various chemical and metallurgical processes.29,30,31) During the mechanical milling, the powder particles are fractured, causing the increment of the contacting area and active reaction point between the iron-containing mineral and reducing agent, which in turn improves the reaction rate.
The reduction time was set as 30 mins and the experimental results are summarized in Fig. 7. Figure 7(a) shows that the metallization degree of the pellet without milling pretreatment increases fast with increasing reduction temperature and the increasing rate becomes slow at the temperature higher than 1150°C due to the formation of liquid slag phase. The liquid phase could prevent direct contact between iron oxide and solid carbon, and the reactivity of iron oxide may also decrease as melting with other components. Therefore, the reduction rate decreases correspondingly. Increasing the substitution ratio of char coal to anthracite coal (Fig. 7(b)) and the adding ratio of Na2CO3 (Fig. 7(c)) can only improve the reduction rate to a certain extent. Figure 7(d) has illustrated that mechanical milling pretreatment of the mixture of copper smelting slag and anthracite coal can significantly increase the pellet metallization degree from 54.5% to 75.5% when the slag-coal mixture is just milled for 30 s and reduced at 1100°C. However, the pellet metallization degree changes little with the further increment of milling time. Based on the aforementioned experimental results, it can be concluded that milling for mechanical activation is the most efficient method to improve the reduction rate of copper smelting slag/coal composite pellet under the present experimental condition.

Effect of different factors on pellet reduction. (a) temperature, (b) coal char addition, (c) Na2CO3 addition, (d) milling for mechanical activation.
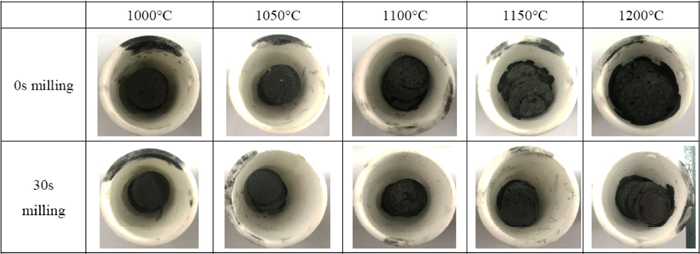
The pellets with and without mechanical milling pretreatment were reduced at temperatures ranging from 1000°C to 1200°C, and the morphologies of the reduced pellets are listed in Fig. 8. For the pellet without mechanical milling, it began to soften at 1100°C, melting a little at 1150°C and melting a lot at 1200°C. The aforementioned phenomenon resulted from the melting of Fe2SiO4, whose melting point is only 1205°C, and the eutectic reaction between Fe2SiO4 and other components (such as FeO, Al2O3, CaO, K2O, Na2O, etc.), which makes the melting point of the slag increasingly lower. If the copper smelting slag composite pellet is reduced in a RHF, two or three layers of green pellets will be laid on the hearth of the furnace and then reduced in static state. So the pellet will directly contact the surface of the hearth. In order to protect the refractory material of the hearth of RHF from erosion in the industrial production process, the furnace heating temperature should be controlled lower than 1100°C. However, the metallization degree of 1100°C reduced pellet is just around 54.5% (30 min), which is not suitable for the production of high grade iron concentrate by magnetic separation technology. Fortunately, the pellet with 30 s milling pretreatment keeps its original shape even reduced at 1200°C. It also can be observed that the volume shrinkage value of pellet with milling is bigger than that of the reduced pellet without milling, which could improve the external heat transfer from the top layer to the second layer when using a multilayer burden bed.32) The metallization degree of pellet with milling is significantly increased during reduction compared with the pellet without milling, and the metallization degree is around 75.5% if the 30 s milled pellet was reduced at 1100°C for 30 min. Therefore, the mechanical milling technology can help protect the hearth of RHF and improve the quality of reduced pellet. Furthermore, the reduction time, furnace heating temperature and energy consumption will also be reduced if the mechanical milling technology can be implemented in the treatment of mixture of copper smelting slag and solid carbon reducing agent at industrial scale.
Morphology of reduced pellets. (Online version in color.)
The microscopic analysis of reduced pellets (0 s and 30 s milling) was performed by SEM-EDS as shown in Fig. 9. The analysis can help understand the reduction process of the pellet and the difference between the pellets with and without milling. At the temperature of 1000°C, the reduced pellets are still in the state of mixture of powdery slag and coal. After mechanical milling, the amount of large-size slag and coal particle has decreased and their distribution becomes more uniform. The metallic iron could not be observed due to the smaller amount. At the temperature of 1050°C, large pore and metallic iron particles begin to appear. The pore size is smaller and the amount of metallic iron particle is more compared with the un-milled sample. At the temperature of 1100°C, the liquid slag phase becomes to appear from the microscopic level for sample with milling, and the amount of metallic iron particles has obviously increased for both of the samples. At the temperature of 1150°C, the slag phase appears in a large quantity, FeO-rich mineral particle has disappeared, and the amount and size of the metallic iron particle continue to increase. At the temperature of 1200°C, the microstructure of the two pellets changes little compared with those reduced at 1150°C. The grain size of single metallic iron particle is generally smaller than 30 μm and the metallic iron particles gathering around the residue coal particles are relatively larger than those far away from the coal particles. Generally speaking, the size of metallic iron particle is closely related to the performance of magnetic separation experiment. However, the SEM image shows that the grain size of metallic iron particle is not large enough even reduced at 1200°C. Hence, the dissociation of metallic iron during the grinding of reduced pellet will be very difficult. The EDS analysis results show that the gangue slag phase contains about 21.77 mass% Fe and the metallic iron phase contains about 0.33 mass% Cu for the 30 s milled pellet reduced at 1200°C. The copper in the copper smelting slag exists mainly in the form of Cu2S and Cu.33) Cu2S can be easily reduced by CO and the reaction abides by the following equation:34)
| (7) |

Microscopic morphology of reduced pellets. (a-1) 0 s, 1000°C, (a-2) 0 s,1050°C, (a-3) 0 s,1100°C, (a-4) 0 s, 1150°C, (a-5) 0 s,1200°C; (b-1) 30 s,1000°C, (b-2) 30 s, 1050°C, (b-3) 30 s,1100°C, (b-4) 30 s,1150°C, (b-5) 30 s, 1200°C. (Online version in color.)
The metallic copper can easily form alloy with metallic iron during the reduction process.
3.3. Iron Oxide Reduction Kinetics AnalysisIn order to reveal the reduction mechanism of the iron oxide in the copper smelting slag and verify the improving effect of mechanical milling on the reduction, the reduction kinetics analysis has been conducted in the present study. The course of iron oxide reduction reaction was expressed in terms of ‘reaction fraction’ (f), which has been defined as weight loss measured at a given time (t) divided by the maximum possible weight loss. It was assumed that only the iron oxide was reduced by solid carbon and CO was the only gaseous product. The maximum possible weight loss included the weight loss of oxygen atom in iron oxide, fixed carbon and volatile, which were contained in the composite pellet.
The variation of reaction fraction with time of the milled and un-milled pellets when reduced at the temperature ranging from 1000°C to 1150°C is shown in Fig. 10. It can be seen that the mechanical milling can improve the reduction rate remarkably and the effect increases with increasing temperature. Generally speaking, the mechanical milling can make reduction temperature decrease by 50°C at least and help the reduction degree reach a higher value.

Variation of reaction fraction during reduction. (Online version in color.)
The reduction of iron oxide has been taken as the first order reaction by many researchers.23) The relation between reduction fraction and rate constant can be expressed as follows,
| (8) |
For a first-order reaction, the reaction fraction curves should be sigmoidal shape. However, the curves in Fig. 10 are almost linear at the beginning stage (within 4 min). It means that the initial reduction process does not follow the first-order rate equation as well as the latter part. The main reason may be the lower temperature of the pellet at the initial reduction stage, which is controlled by heat transfer. This phenomenon is a natural process which may not be avoided for a pellet of this size during reduction. It also can be seen that the initial reaction fraction within 4 min of pellet with milling is larger than the pellet without milling at each temperature. The reason may be as follows. On the one hand, the pellet with milling is more compacted, and heat transfers rate is higher in it. So, the reduction is improved. On the other hand, the contacting area between the iron-containing mineral and reducing agent is increased due to mechanical milling, and the reduction rate is correspondingly increased.
Introducing the ‘f’ and ‘t’ of different temperatures into Eq. (8), the reduction rate constants of copper smelting slag/coal composite pellet with and without milling can be obtained through linear fitting, which are shown in Fig. 11. The data at the beginning of the reduction within 4 min are not used to avoid the influence on the calculation accuracy of activation energy. It can be observed that the -ln(1-f) vs. time curves at all the temperatures are in good linearity throughout the reduction process as the linearly dependent coefficients for all the curves are all bigger than 0.95, and the fitting linearity of the milled pellet curve is even much better. The value of rate constant increases with increasing temperature. Mechanical milling will also increase the value of rate constant at each temperature, which agrees well with the reduction experimental results.

Linear fitting result of -ln(1-f) vs. time curves. (a) 0 s milling, (b) 30 s milling. (Online version in color.)
The apparent activation energy can be calculated out using the rate constants by the Arrhenius equation, which is listed as Eq. (9).
| (9) |

Arrhenius plots of the rate constants for the reduction. (a) 0 s milling, (b) 30 s milling. (Online version in color.)
| Reaction | Ea/kJ/mol |
|---|---|
| (1/4)Fe3O4+CO=(3/4)Fe+CO2 (T<570°C) | 73.6 |
| Fe3O4+CO=3FeO+CO2 (T>570°C) | 73.6 |
| FeO+CO=Fe+CO2 | 69.4 |
| C+CO2=2CO | 221.8 |
In order to evaluate the effect of mechanical milling on the production of high T.Fe content concentrate from reduced copper smelting slag/coal composite pellet, the magnetic separation experiment was performed at laboratory scale in the present work. The pellet without milling pretreatment reduced at 1100°C for 30 min and the pellets with 30 s milling pretreatment reduced at 1100°C, 1150°C and 1200°C for 30 min were used in the magnetic separation experiment, as demonstrated in Fig. 13. The Fe recovery rates of all the concentrates obtained from the pretreated pellets with milling are basically similar and much higher than that from the pellet without milling. At the temperature of 1100°C, the T.Fe content of concentrate of the pellet with milling is inferior to that of the pellet without milling. However, the yield of the concentrate of the milled pellet reduced at 1100°C is much better, which may be due to the lower degree of dissociation between metallic iron particles and gangue minerals. With further increasing of reduction temperature for the pellets with milling, the T.Fe content, metallization degree and Fe recovery rate of the concentrate are gradually improved, which are better than those of the un-milled pellet reduced at 1100°C except for yield. It is well known that T.Fe content, metallization degree and Fe recovery rate are more important than yield. Totally speaking, the quality of the iron concentrate from the milled pellet reduced at 1200°C is much better than the concentrates obtained under other experimental conditions. For the concentrate obtained under aforementioned experimental conditions, the T.Fe content, metallization degree and Fe recovery rate are 86.9%, 93.9% and 95.1%, respectively. For pellet with the pretreatment of mechanical milling, the optimal reduction temperature should be better within the range from 1100°C to 1200°C. In other researches,16,17) the CaO has to be added to improve the reduction of fayalite and the reduction temperature should be higher than 1200°C to obtain similar reduction-magnetic separation performance. It can be concluded that mechanical milling is very effective during the iron enrichment from copper smelting slag by direct reduction and magnetic separation process. The chemical composition of the iron concentrate from the pellet milled for 30 s and reduced at 1200°C is listed in Table 4.

Properties of separated concentrate under different parameters. (a) yield, (b) T.Fe content, (c) metallization degree, (d) recovery rate of Fe.
| T.Fe | MFe | FeO | MgO | SiO2 | Al2O3 | CaO | ZnO | K2O | Na2O | Pb | Cu |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 86.9 | 81.6 | 6.81 | 0.37 | 8.32 | 1.33 | 0.64 | 0.036 | 0.22 | 0.18 | <0.02 | 0.37 |
The mass balance analysis of the process from raw copper smelting slag-coal mixture to iron concentrate from the pellet milled for 30 s and reduced at 1200°C has been carried out. The total removal rate of gangue mineral (i.e. except for metallic iron or iron-containing oxide) of the aforementioned process reaches to about 88.3%. The removal rates of Zn, K, Na and Cu are listed in Fig. 14, respectively. Zn has the largest removal rate, which is about 99.3%. The removal rates of different elements decrease in the sequence of Zn>K>Na>Cu. The removal rate of Cu is the smallest because the Cu cannot volatilize into gas atmosphere and most metallic copper will dissolve into metallic iron during the reduction process. The iron concentrate cannot meet the requirements of blast furnace ironmaking for Zn, K, Na and Cu in the burden. A new smelting technology for the utilization of the iron concentrate obtained from direct reduction-magnetic separation of copper smelting slag should be developed. Furthermore, the theoretical Cu concentration in the smelted metal obtained from the iron concentrate is about 0.43 mass% and the molten metal can be used to produce Cu-containing high-quality weathering steel at lower cost.36)

Removal rate of typical constituents from the beginning of copper smelting slag.
SEM-EDS has been used to analyze the microstructure and chemical content of the concentrate obtained by magnetic separation of the pellet (30 s milling) reduced at 1200°C for 30 min. As shown in Fig. 15, the large-size metallic iron particles have been basically separated apart from the slag gangue. However, a certain amount of gangue particles still exist in the concentrate and most of the gangue particles combine with the metallic iron particles due to low degree of dissociation. The poor dissociation may result from the small grain size of metallic iron particles in the final reduced pellet. The aforementioned results indicate that the difficulty in dissociating slag gangue from metallic iron and the difficulty in separation after dissociation are the major causes of lower T.Fe grade and metallization degree of the concentrate. In order to obtain concentrate of higher quality, the grain size of the ground reduced pellet should be much smaller than 74 μm before being subjected to magnetic separation. Furthermore, research on the technology that can improve the growth of metallic iron particles during reduction will be also of great significance in the future.

Microscopic morphology of concentrate from 1200°C reduced pellet (30 s milled). (Online version in color.)
Generally, the elements, such as Zn, Pb, K and Na, in the metallurgical dust will transfer into the atmosphere during the high-temperature reduction process of the Fe-rich composite pellet.37) The metal vapors will then be oxidized in the flue gas and form fine dust and finally be captured by the bag filter. The so-called secondary dust can be used as valuable raw material for the zinc production, which is of great significance for the recycling of zinc resource in the modern society. The chemical analysis reveals that the copper smelting slag contains 2.46 mass% ZnO. Most researchers in this field mainly focus on the reduction behavior of iron oxide and the recovery of iron by direct reduction-magnetic separation or the recovery of non-ferrous metals by acid leaching.20,21) In the present work, the volatilization and characteristics of the dust were investigated during the reduction of the copper smelting slag. In this part, the green pellets were made by the raw materials milled for 30 s and the dry green pellet was heated at 1200°C for 30 min. The off gas from the furnace tube was filtered by a quartz membrane filter. The picture of obtained dust is shown in Fig. 16. It can be seen that the captured dust is yellowish white with very fine particle size. Chemical composition of the dust is listed in Table 5. The dust mainly consists of 70.17 mass% Zn and 11.99 mass% Pb. The X-ray diffraction analysis in Fig. 17 indicates that the Zn in the dust exists in the form of ZnO. If the Zn is converted to ZnO, the ZnO content in the dust can increase to 87.44 mass%. The content of Zn is the main index of zinc ore. For the Igrade zinc ore, the content of Zn should be higher than 55.00 mass%.38) So the captured dust can meet the requirements of Igrade zinc ore. The Zn content in the dust obtained in the present work is 10 mass% higher than that by other researchers under similar condition.39) The possible reasons are as follows. On the one hand, the compared study is carried out in an industrial scale RHF and the dust contains some gangue minerals, which might result from breakage of the composite pellet. On the other hand, the reduction of ZnO in the copper smelting slag has been improved due to mechanical milling because the Zn content in the compared study is still lower even taking no account of the gangue minerals. Based on the arithmetic average of the three tests, the dust yield under the present experimental condition is 1.49%. As mentioned above, approximately 24.6 million tons of copper smelting slags are generated in each year all over the world. It can be roughly concluded that 0.37 million ton high-quality Zn-rich dust can be obtained, which contains 0.26 million ton Zn. It will be meaningful for the sustainable development of global zinc market. The microscopic structure of the captured dust analyzed by SEM-EDS is shown in Fig. 18. It can be observed that the grain size of the largest ZnO particle is less than 3 μm and most of the particles are much finer than 3 μm. The agglomerate larger than 9 μm visible on the right side of the SEM image should result from the aggregation of ultra-fine captured dust particles. During the preparation of captured dust sample for SEM analysis, the dust powder was mixed with liquid resin and then the slime mixture solidified. Generally, some dust powder could not completely be mixed with the viscous liquid resin into uniform state, and therefore looks like agglomerate on the SEM image.

Macroscopic and microscopic morphology of captured dust. (Online version in color.)
| Zn | Pb | K | Na | S |
|---|---|---|---|---|
| 70.17 | 11.99 | 0.17 | 0.29 | 0.29 |

XRD pattern of captured dust.

Microscopic structure image and EDS analysis of captured dust. (Online version in color.)
(1) The reduction of fayalite is the main task during the reduction of copper smelting slag due to its worse reducibility. Milling for mechanical activation is an efficient method to improve the reduction of copper smelting slag. The pellet metallization degree increases from 54.5% to 75.5% when the slag-coal mixture is milled for 30 s and reduced at 1100°C, which can help restrain slag melt formation. Apparent activation energy increases from 96.1 kJ/mol to 153.5 kJ/mol when the mechanical milling is applied and the rate controlling step of reduction shifts from surface reduction to solid carbon gasification. The Cu2S can be easily reduced and form alloy with metallic iron during the reduction process.
(2) With further increasing of reduction temperature, the T.Fe content, metallization degree and Fe recovery rate of the concentrate of milled pellets are gradually improved and better than those of un-milled pellets reduced at 1100°C except for yield. The quality of iron concentrate obtained from the milled pellet reduced at 1200°C is highest, whose T.Fe content, metallization degree and Fe recovery rate are 86.9%, 93.9% and 95.1%, respectively. The removal rates of different elements during direct reduction-magnetic separation decrease in the sequence of Zn>K>Na>Cu. Finer grinding size is necessary to obtain higher-quality concentrate during magnetic separation.
(3) Yellowish white dust with very fine particle size smaller than 3 μm can be captured from the flue gas during the reduction of the composite pellet at 1200°C. The dust mainly consists of 70.17 mass% Zn and 11.99 mass% Pb. The Zn in the dust exists in the form of ZnO. The Zn content in the dust obtained in the present work is much higher than that by other researchers under similar condition. The dust can meet the requirements of Igrade zinc ore.
The authors would like to express their gratitude for the financial support of the National Natural Science Foundation of China (51804024) and the Fundamental Research Funds for the Central Universities (FRF-IC-20-09).