2022 Volume 62 Issue 6 Pages 1099-1105
2022 Volume 62 Issue 6 Pages 1099-1105
The viscosity of 30%CaO-30%SiO2-15%Al2O3-5%MgO-10%Na2O-10%CaF2-xCe2O3 (mass%, x = 0, 5, 10, 15) were measured by the rotating column method, and then the viscosity and the Tbr (break temperature) of the mold flux were analyzed based on the results. Meanwhile, the structural characteristics of the mold flux were investigated using Raman spectroscopy and XRD (X-ray diffraction). The results show that Ce2O3 predominantly destroys the silicate network structure at high temperature, reduces the polymerization degree of the mold flux, simplifies the high-temperature structure of the mold flux, and reduces the friction resistance of viscous flow. From the apparent phenomenon, the viscosity of the mold flux decreases at high temperature. In addition, with the increase of Ce2O3 content in the mold flux, Ca4Si2O2F changes to Ce9.33(SiO4)6O2, which enhances the crystallization ability of the mold flux and increases the Tbr of the mold flux.
In the process of continuous casting, mold flux can effectively realize heat transfer and lubrication between slab and mold flux, absorb floating inclusions in molten steel and prevent secondary oxidation of molten steel. Therefore, mold flux has already become the key functional material in continuous casting process.1,2) Generally, the traditional mold flux for continuous casting is a mixture composed of CaO and SiO2 as the main components, adding a certain amount of fluxes such as Na2O and CaF2, and carbonaceous material for adjusting melting rate. The composition is shown in A region of Fig. 1.3,4) When casting steel grades containing easily-oxidizable elements, the high content of SiO2 in the mold flux is easily reduced by the active metal elements (such as Eq. (1)) to form high melting point phases, such as CaAl4O7, CaTiO3, etc., which will increase the melting point and viscosity of the mold flux, and then affect the smooth operation of the continuous casting process.5,6,7)
| (1) |
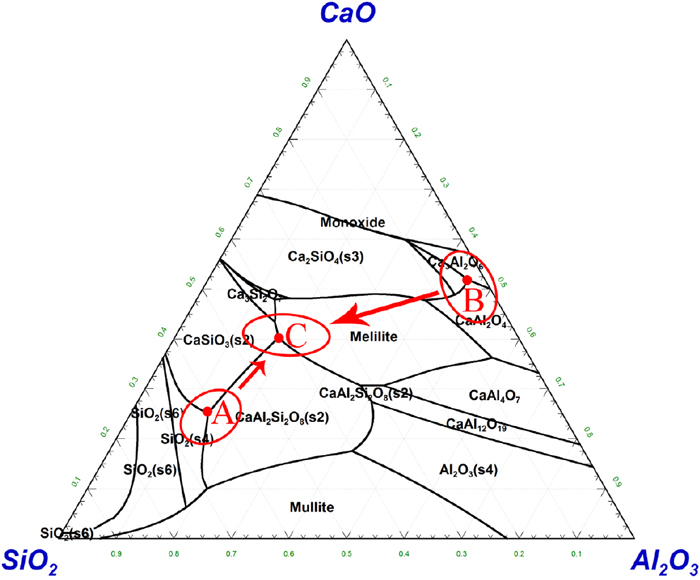
The phase diagram of CaO–SiO2–Al2O3. (Online version in color.)
In order to solve the problem of elements oxidation, scholars proposed to use CaO–Al2O3-based mold flux,8,9,10) and its composition is shown in B region of Fig. 1. The composition of the mold flux is easy to form gehlenite with high melting point, which makes the glass performance of the mold flux worse, thus affects the lubrication of mold flux and deteriorates the surface quality of slab.11,12,13) Therefore, it is necessary to enhance the glass performance of mold flux by adding fluxes such as B2O3. However, B2O3 reacts more easily than SiO2, and the reaction described in Eq. (1) will still occur.14)
In view of the problems of element oxidation and lubrication in the continuous casting process, scholars have developed a low-activity mold flux based on CaO–SiO2–Al2O3-based slag system,15) and its composition is shown in C region of Fig. 1. The main precipitated phases in this region are melilite, CaAl2Si2O8 and CaSiO3. At this time, SiO2 can effectively combine with other components, so the activity of SiO2 in C region is lower than that in A region, and the chemical reaction between slag and steel at the interface is weak. At the same time, the content of SiO2 in the mold flux is high, which can effectively ensure the lubricating ability of the mold flux.
Adding stable oxide Ce2O3 to the CaO–SiO2–Al2O3-based mold flux to form Ce2Si2O716) can further control the activity of SiO2. Anacleto et al.17) proposed that Ce2O3 could reduce the activity of SiO2 in the CaO–SiO2–Ce2O3 system during the study of the sulfur partition between CaO–SiO2 slags and carbon-saturated iron at 1500°C. But they did not provide any relevant proof. At present, there are few reports on the effect of Ce2O3 on the viscosity and structure of CaO–SiO2–Al2O3-based mold flux. In the study of similar slag systems, Jeong et al.18) and Kim et al.19) investigated the network structure of the MnO–SiO2–Al2O3–Ce2O3 slag system. When Ce2O3 was added to the slag (M/S = 2.2), the [AlO4]−: 0.5Mn2+ units were transformed to [AlO6]3−: Ce3+ units by employing free oxygen. However, structural changes were not observed despite the addition of Ce2O3 to the slag (M/S = 0.9). Qi et al.20) found a similar situation in the study of the CaO–Al2O3–Li2O–Ce2O3 slag, where the content of Ce2O3 increased from 0 to 10 wt pct, the relative fraction of [AlO4]-tetrahedron decreased and the relative fraction of [AlO6]-octahedron increased obviously.
The viscous flow behavior of 30%CaO-30%SiO2-15%Al2O3-5%MgO-10%Na2O-10%CaF2-xCe2O3 (mass%, x = 0, 5, 10, 15) system was studied in this paper. The effects of Ce2O3 content on the viscosity and Tbr of the mold flux were discussed in detail. At the same time, its mechanism was clarified by Raman spectroscopy and XRD.
The mold flux used in the experiment is Ce2O3 with different quality added to 30%CaO-30%SiO2-15%Al2O3-5%MgO-10%Na2O-10%CaF2. The chemical composition ratio of the mold flux is shown in Table 1. Analytical grade CaO, SiO2, Al2O3, MgO, Na2CO3 (instead of Na2O), CaF2 and Ce2C3O9 (instead of Ce2O3) were used as raw materials (purity > 99%), and CaO, MgO and Ce2C3O9 powders were calcined at 873 K for 2 h to remove water in the experiment.
| Sample | CaO | SiO2 | Al2O3 | MgO | Na2O | CaF2 | Ce2O3 |
|---|---|---|---|---|---|---|---|
| S0 | 30.0 | 30.0 | 15.0 | 5.0 | 10.0 | 10.0 | 0.0 |
| S1 | 28.5 | 28.5 | 14.2 | 4.8 | 9.5 | 9.5 | 5.0 |
| S2 | 27.0 | 27.0 | 13.5 | 4.5 | 9.0 | 9.0 | 10.0 |
| S3 | 25.5 | 25.5 | 12.7 | 4.3 | 8.5 | 8.5 | 15.0 |
| S4 | 24.0 | 24.0 | 12.0 | 4.0 | 8.0 | 8.0 | 20.0 |
The viscosity of mold flux was measured by rotation method.21,22,23) The experimental equipment is BCT1700, which conforms to the standard methods of American ASTMC 965 and ASTMC 1276. The measurement accuracy of the testing instrument is ±1%, and the reproducibility is within ±0.2%. In the experiment, molybdenum crucible (inner diameter 40 mm) and molybdenum rotor (cylindrical section size 12 mm in diameter and 10 mm in height) were used, and the angles of the top and tail of the rotor were 120°. The schematic diagram of the experimental equipment is shown in Fig. 2.

Schematic diagram of experimental equipment. (Online version in color.)
Firstly, the experimental equipment was calibrated with standard silicone oil at room temperature and 100 r·min−1. Secondarily, the sample was heated to 1773 K (1500°C) for 30 mins to obtain a uniform melt. Then the rotor was slowly immersed in the crucible, stopped at 10 mm from the bottom of the crucible, kept concentric with the crucible, and stirred evenly for 10 mins. At last, the viscosity of the mold flux was measured by cooling temperature. The rotor speed was 100 r·min−1, the cooling rate was 10°C·min−1, and the viscosity value reached 10 Pa·s at the end of the measurement. During the whole experiment process, high purity argon was continuously introduced for protection, and the argon flow rate was 0.2 NL·min−1.
2.3. Raman Spectroscopy MeasurementsThe mold flux was heated to 1773 K (1500°C) in the furnace based on MoSi2 heating elements, then quenched on water-cooled copper plate, and quenched sample was crushed to less than 74 μm. The XRD results showed that the quenched samples were amorphous without characteristic peaks, as shown in Fig. 3. The structural information of glass samples obtained from quenching was detected by Raman spectroscopy (LabRAM HR evolution, Horiba, jobin, Yvon, France). The experiment was carried out at 25°C, the excitation wavelength was 532 nm, the light source was a semiconductor laser with power of 1 mw, the exposure time was 10 s, and the continuous scanning mode was adopted. The measurement frequency band range was 200–1600 cm−1, the scanning time was 6 times, and the spectral resolution was less than or equal to 0.65 cm−1. Finally, the peak fitting of Raman spectra in the range of 400–1400 cm−1 was performed by using Gaussian function.

Water quenching XRD pattern of the mold fluxes at 1773 K. (Online version in color.)
The average value of the three viscosity tests was used as the viscosity of the mold flux at different temperatures, and the viscosity-temperature curves of the mold flux with different Ce2O3 content are shown in Fig. 4.

Viscosity-temperature curves of the mold fluxes with different Ce2O3 content. (Online version in color.)
It can be seen from Fig. 4 that the viscosity of samples S0–S4 decreases with the increasing temperature. When the temperature is lower than the Tbr, the viscosity of the mold flux rises rapidly, reaching 10 Pa·s in a narrow temperature range, the melt changes from Newtonian fluid to non-Newtonian fluid,24,25) and the fluidity and stability of the mold flux become poor. When T ≥ 1670 K, the content of Ce2O3 has less effect on the viscosity of the mold flux. The viscosity of each mold flux changes gently with temperature and the viscosity value shall not exceed 0.03 Pa·s. At this time, the viscosity of the mold flux decreases with the increase of Ce2O3 content in the mold flux. In addition, the viscosities at 1573°C are listed in Table 2.
| Sample | η (Pa·s) | Tbr (K) |
|---|---|---|
| S0 | 0.29 | 1418 |
| S1 | 0.30 | 1467 |
| S2 | 0.33 | 1472 |
| S3 | 0.35 | 1486 |
| S4 | 0.40 | 1507 |
The relationship between the activation energy and temperature may be illustrated using the Arrhenius equation, as shown in Eq. (2).26)
| (2) |
Where, η is the viscosity of the mold flux (Pa·s), A is the pre-exponent factor; Ea is the activation energy for the viscous fluid (J·mol−1), R is the gas constant (8.314 J·mol−1·K−1), and T is the absolute temperature (K). The results are shown in Fig. 5 and the corresponding break temperatures of the mold flux are listed in Table 2.

Relationship between lnη and 1/T. (Online version in color.)
It can be seen from Table 2 that the viscosity of the mold flux for samples S0–S4 increases from 0.29 to 0.40 Pa·s with the increase of Ce2O3 content at 1573 K, which indicates that when the temperature is lower than the Tbr, the viscosity of the mold flux increases with the increase of Ce2O3 content in the mold flux. The lowest Tbr of mold flux is S0 (1433 K), followed by S1 (1461 K), S2 (1472 K), S3 (1483 K) and S4 (1512 K). With the increase of Ce2O3 content in the mold flux, the Tbr of the mold flux increases.
Tbr is an important parameter of mold flux.27,28) When the temperature is lower than Tbr, a large number of crystalline phases start to precipitate from mold flux, and the viscosity of mold flux will increase rapidly. This indicates that with the increase of Ce2O3 content in the mold flux, the crystallization ability of the mold flux increases and the lubricating performance decreases.
The content of Ce2O3 in the mold flux should not exceed 15%. On the one hand, when the content of Ce2O3 in the mold flux is 15%, the obvious inflection point appears in the viscosity curve of the mold flux, which indicates that the mold flux belongs to the alkaline slag system, as shown in Fig. 4. On the other hand, the Tbr of mold flux exceeds 1473 K, which tends to lead to a sharp thinning of liquid slag film and insufficient lubrication of the billet, which is unfavorable for continuous casting production.
3.2. Effect of Ce2O3 on High-Temperature Structure of Mold FluxeThe high-temperature structure of slag directly influences the viscosity of the slag, and the network structure formed by the different oxides act differently at high temperature. Ce2O3, as a basic oxide, will become a network modifier like CaO.29) At high temperature, it will break the bridge oxygen bond and form non-bridge oxygen bond, which will lead to the disintegration of slag network structure and the decrease degree of polymerization, thus reducing the viscosity of slag.
In order to understand the polymerization degree of slag at high temperature, NBO/T is often used for characterization, which is expressed as the ratio of the number of non-bridged oxygen atoms to the number of tetrahedral coordination atoms in the slag. The calculation equation is shown in Eq. (3).30,31)
| (3) |
Where, NBO is the non-bridge oxygen number, T is the number of quaternary coordinated cations, x is the mole fraction of the component. The NBO/T values of the samples S0–S4 are shown in Fig. 6.

Change of NBO/T with Ce2O3 addition in the mold flux.
It can be seen from Fig. 6 that when Ce2O3 component is added to the mold flux with similar mass ratio of other components, NBO/T increases from 2.02 to 2.18, which indicates that the depolymerization degree of the mold flux structure increases. This is due to the fact that Ce2O3, as a network modifier, reduces the number of quaternary coordination cations, improves the polymerization degree of the mold flux, and simplifies the high-temperature structure of the mold flux. This will decrease the diffusion resistance of the mold flux components and the energy barrier of crystallization,32) reduce the viscosity of mold flux and improve the crystallization tendency of the mold flux.
In order to determine the high-temperature structure of mold flux, the structures of samples S0–S4 at 1773 K are analyzed by Raman spectroscopy, as shown in Fig. 7. As can be seen from Fig. 7, the peaks of Raman spectrum are concentrated in three main regions. Among them, the maximum peak value of Raman spectrum curve is located at 800–1100 cm−1, which is the region of symmetrical stretching of [Si–O], and the peak value shifts from 906 to 863 cm−1 with the increase of Ce2O3 content in the mold flux. It indicates that with the addition of Ce2O3 in the mold flux, the Raman spectrum peaks shift to lower frequency, and the structure of [Si–O] is destroyed to a certain extent, which reflects the role of Ce2O3 as a network modifier in the microstructure of the mold flux. In contrast, the characteristic peaks of Raman spectra at 571 cm−1 and 690 cm−1 are not shifted, indicating that Ce did not participate in the [Al–O] structure. This indicates that Ce2O3 will preferentially destroy the silicate network structure.

Raman spectra of mold fluxes with different Ce2O3 content at 1773 K. (Online version in color.)
To further analyze the specific effect of Ce2O3 on [Si–O] structure, the signals in the high frequency region 800–1100 cm−1 of Raman in Fig. 7 are fitted, and the results are shown in Fig. 8. It can be seen from the figure that the fitting peaks in the region of 800–1100 cm−1 are 857–852, 903–899, 972–969 and 1039 cm−1 respectively. According to Raman spectrum analysis, the fitting peaks correspond to Q0, Q1, Q2 and Q3 structural units in [Si–O] structure respectively, as shown in Table 3. By comparing the shift of fitted peaks, it can be seen that the fitted peak shifts to lower frequency with the increase of Ce2O3 content. At this time, the region of these characteristic peaks also changes differently.

Raman deconvolution results of (a) sample S0, (b) sample S1, (c) sample S2, (d) sample S3, and (e) sample S4. (Online version in color.)
| Raman bands | Raman shift (cm−1) | Reference |
|---|---|---|
| Symmetric stretching vibration | 1040–1060 | 33,34,35,36,37,38,39 |
| Symmetric stretching vibration | 950–980 | 33,34,35,36,37,38,39 |
| Symmetric stretching vibration | 900–920 | 33,34,35,36,37,38,39 |
| Symmetric stretching vibration | 850–880 | 33,34,35,36,37,38,39 |
| Symmetric stretching vibration | 600–700 | 40,41,42,43 |
| Symmetric stretching vibration | 400–600 | 44,45,46 |
The relative content of different structural units in the slag can be inferred from the corresponding Raman band area, and then the mole fraction of species in the silicate network
| (4) |
Where, Xn is mole fraction (n ranges from 0 to 3), θn is Raman scattering coefficient and An is Raman band area. Although the exact value of θn is still unknown, as an alternative, considering that θn is a constant and only determined by the

Relative area fractions of different structural units in the mold flux under different Ce2O3 mass fractions. (Online version in color.)
It can be seen from Fig. 9 that when the content of Ce2O3 in the mold flux increases from 0 to 20%, the contents of Q0 and Q1 in the [Si–O] structure gradually increase, the content of Q2 gradually decreases, and the content of Q3 becomes 0, that is, with the increase of Ce2O3 content in the mold flux the relative fraction of [Si–O] tetrahedron (network forming body) in the mold flux decreases.
It is generally believed that the change of relative content and conversion of various structural units will affect the degree of polymerization of slag.49) The reason for the depolymerization of network structure may be that Ce2O3 is decomposed into Ce3+ and O2− in the mold flux, O2− dissociated from Ce2O3 participates in [Si–O] structure through Eq. (5).
| (5) |
Therefore, Ce2O3 can be used as a network modifier, which will destroy the bridge oxygen bond and form non-bridge oxygen bond at high temperature. The disintegration of the network structure simplifies the high-temperature structure of the mold flux and reduces the friction resistance to the viscous flow of the mold flux. From the apparent phenomenon, the viscosity of the mold flux decreases at high temperature.
3.3. Effect of Ce2O3 on Crystallization of Mold FluxThe microstructure of melt affects the crystallization behavior of the mold flux and the precipitation of crystalline phase also affects the crystallization temperature of the mold flux. Therefore, in order to explore the effect of Ce2O3 on the crystallization behavior of the mold flux, XRD analysis was carried out on the low-temperature water quenched slag of samples S0–S4, as shown in Fig. 10.

XRD of different Ce2O3 mass fractions. (Online version in color.)
It can be seen from Fig. 10 that the main crystalline phases in the mold flux are Ca4Si2O2F, Na6Al4Si4O17, Ca2Al2SiO7 and Ca3MgSi2O8. When the mass fraction of Ce2O3 in the mold flux increases to 10%, Ce9.33(SiO4)6O2 begins to appear in the mold flux. With the increase of Ce2O3 content in mold flux, the peak value of Ce9.33(SiO4)6O2 increases, and the peak values of Ca4Si2O2F and Na6Al4Si4O17 decrease significantly. This proves that Ce2O3 participates in [Si–O] structure, forms cerium silicate, and enhances the crystallization ability of mold flux. In addition, since the melting point of Ce9.33(SiO4)6O2 (1770°C)16) is higher than Ca4Si2O2F (1407°C),50) the Tbr of mold flux will increase with the increase of Ce2O3 content in mold flux.
In this paper, the viscosity, structure and crystallization of 30%CaO-30%SiO2-15%Al2O3-5%MgO-10%Na2O-10%CaF2-xCe2O3 (mass%, x = 0, 5, 10, 15) were studied. The conclusions are as follows:
(1) The results of viscosity experiment show that when Ce2O3 content increases from 0% to 20% in the mold flux, the high-temperature viscosity of mold flux decreases, and the Tbr increases from 1433 K to 1515 K. It is more appropriate to add 15% Ce2O3 to mold flux.
(2) The results of NBO/T and Raman spectra show that with the increase of Ce2O3 content in the mold flux, the depolymerization degree of the mold flux structure increases and the high-temperature structure becomes simpler. The peak of Raman spectrum shifts from 906 cm−1 to 863 cm−1, and the addition of Ce2O3 will preferentially destroy the silicate network structure in the mold flux.
(3) The results of XRD show that when content of Ce2O3 in mold flux increases to 10%, Ca4Si2O2F in mold flux changes to Ce9.33(SiO4)6O2, and the crystallization ability of mold flux increases.
(4) Ce2O3 can be used as a network modifier, which will destroy the bridge oxygen bond and form non-bridge oxygen bond at high temperature. The disintegration of the network structure simplifies the high-temperature structure of the mold flux, reduces the friction resistance of the viscous flow of the mold flux. From the apparent phenomenon, the viscosity of the mold flux decreases at high temperature.
This work was supported by the National Natural Science Foundation of China [U1760206].